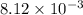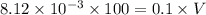Chemistry, 16.03.2020 23:29 brandon56238
Consider the following four titrations: i. 100.0 mL of 0.10 M HCl titrated with 0.10 M NaOH ii. 100.0 mL of 0.10 M NaOH titrated with 0.10 M HCl iii. 100.0 mL of 0.10 M CH3NH2 titrated with 0.10 M HCl iv. 100.0 mL of 0.10 M HF titrated with 0.10 M NaOH Rank the titrations in order of increasing volume of titrant added to reach the equivalence point.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 14:10
Can smoke be transformed into liquid or used as energy or both?
Answers: 2

Chemistry, 21.06.2019 20:30
What problem would a person have if the nucleic acid in one of his or her cells were damaged?
Answers: 2

Chemistry, 22.06.2019 01:00
Which type of orbits are found in the principal energy level n = 2 a - s b - s, f c - s, d d - s, p e - s, p, d
Answers: 1

Chemistry, 22.06.2019 08:30
Which common material is an example of a polymer? (25 pts) a. steel b. plastic c. petroleum d. rubbing alcohol
Answers: 2
You know the right answer?
Consider the following four titrations: i. 100.0 mL of 0.10 M HCl titrated with 0.10 M NaOH ii. 100....
Questions

English, 01.04.2020 01:06


Mathematics, 01.04.2020 01:06


Mathematics, 01.04.2020 01:06

Mathematics, 01.04.2020 01:06

Mathematics, 01.04.2020 01:06

Mathematics, 01.04.2020 01:06

Geography, 01.04.2020 01:06


Mathematics, 01.04.2020 01:06


Mathematics, 01.04.2020 01:07

Mathematics, 01.04.2020 01:07



Mathematics, 01.04.2020 01:07

Mathematics, 01.04.2020 01:07


Computers and Technology, 01.04.2020 01:07

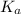 values of
values of 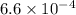 and
and 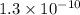 respectively.
respectively.
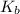 values of
values of 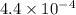 and
and 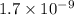 respectively.
respectively.
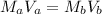
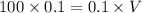
![[OH]^{-} = \sqrt{4.4 \times 10^{-4} \times 0.1}](/tpl/images/0549/5712/43a1f.png)
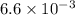
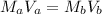
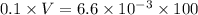
![[H]^{+} = \sqrt{6.6 \times 10^{-4} \times 0.1}](/tpl/images/0549/5712/8b1ed.png)