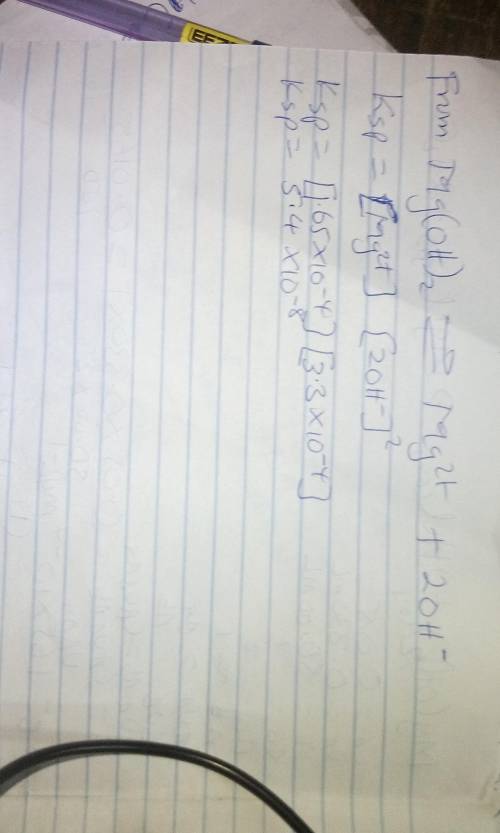Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:00
The pressure in a fluid is affected by which characteristics of that fluid
Answers: 1

Chemistry, 22.06.2019 11:00
Imagine that twenty i.u.’s of enzyme z were catalyzing the above reaction for one minute, under vmaxconditions, in a 3.00 ml assay volume. the assay is buffered with 20 mm phosphate buffer, ph 7.60. what will the ph be at the end of that one minute?
Answers: 2

Chemistry, 22.06.2019 11:30
For each of the following compounds, decide whether the compound's solubility in aqueous solution changes with ph. if the solubility does change, pick the ph at which you'd expect the highest solubility. you'll find ksp data in the aleks data tab. compounds does solubility change with ph
Answers: 3

Chemistry, 22.06.2019 18:10
Given the following at 25c calculate delta hf for hcn (g) at 25c. 2nh3 (g) +3o2 (g) + 2ch4 (g) > 2hcn (g) + 6h2o (g) delta h rxn= -870.8 kj. delta hf=-80.3 kj/mol for nh3 (g), -74.6 kj/mol for ch4, and -241.8 kj/mol for h2o (g)
Answers: 1
You know the right answer?
In an experiment similar to the one described in this procedure, a saturated solution of Mg(OH)2 was...
Questions


Mathematics, 25.02.2021 04:00

French, 25.02.2021 04:00


Mathematics, 25.02.2021 04:00

Mathematics, 25.02.2021 04:00

Chemistry, 25.02.2021 04:00




Mathematics, 25.02.2021 04:00

History, 25.02.2021 04:00

Biology, 25.02.2021 04:00

Geography, 25.02.2021 04:00

Social Studies, 25.02.2021 04:00


Mathematics, 25.02.2021 04:00

Physics, 25.02.2021 04:00

Mathematics, 25.02.2021 04:00

Mathematics, 25.02.2021 04:00