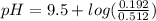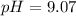Chemistry, 16.03.2020 23:01 thanks5640
A buffer solution contains 0.353 M ammonium bromide and 0.352 M ammonia. If 0.0200 moles of hydrochloric acid are added to 125 mL of this buffer, what is the pH of the resulting solution ? (Assume that the volume change does not change upon adding hydrochloric acid)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:00
How many liters of water vapor can be produced if 108 grams of methane gas (ch4) are combusted at 312 k and 0.98 atm? show all work. pls ! will mark as brainliest
Answers: 1


Chemistry, 22.06.2019 16:30
How many grams of mgbr2 are needed to produce 75g or metal?
Answers: 1

Chemistry, 23.06.2019 04:00
If you are told to get 100 ml of stock solution to use to prepare smaller size sample for an experiment, which piece of glassware would you use?
Answers: 3
You know the right answer?
A buffer solution contains 0.353 M ammonium bromide and 0.352 M ammonia. If 0.0200 moles of hydrochl...
Questions

Mathematics, 27.06.2019 23:30


Mathematics, 27.06.2019 23:30


Chemistry, 27.06.2019 23:30


Mathematics, 27.06.2019 23:30

Social Studies, 27.06.2019 23:30

Mathematics, 27.06.2019 23:30

Mathematics, 27.06.2019 23:30

English, 27.06.2019 23:30

Health, 27.06.2019 23:30

Mathematics, 27.06.2019 23:30


World Languages, 27.06.2019 23:30

Mathematics, 27.06.2019 23:30

Mathematics, 27.06.2019 23:30

English, 27.06.2019 23:30


English, 27.06.2019 23:30

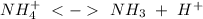
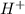 of the hydrochloric acid (
of the hydrochloric acid (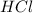 ) will interact with the base of the buffer system (
) will interact with the base of the buffer system (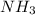 ) to produce more acid (
) to produce more acid (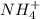 ), so:
), so: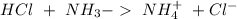
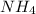 will increase. The next step then would be the calculation of the moles of the acid and base in the buffer system. So:
will increase. The next step then would be the calculation of the moles of the acid and base in the buffer system. So: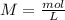
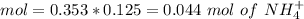
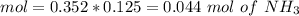
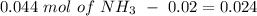
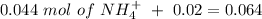
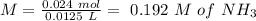
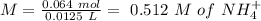
![pH=p{ K }_{ a }+log(\frac { { [A }^{ - }] }{ [HA] } )](/tpl/images/0549/5131/c1f49.png)