
Chemistry, 16.03.2020 21:17 Mariela2699
Determine the equilibrium constant, Kp, for the following reaction, by using the two reference equations below: 2 NO(g) + O2(g) ⇌ 2 NO2(g) Kp = ? References: N2(g) + O2(g) ⇌ 2 NO(g) Kp = 2.3 ´ 10–19 ½ N2(g) + O2(g) ⇌ NO2(g) Kp = 8.4 ´ 10–7

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 19:30
The molecular formula for caffeine is cshion402. which of the following elements is not found in caffeine?
Answers: 1

Chemistry, 21.06.2019 23:30
Why do you suppose the structural polysaccharide cellulose does not contain branches? why do you suppose the structural polysaccharide cellulose does not contain branches? branches in the molecule would generate side chains that would almost certainly make it difficult to pack the cellulose molecules into globules, thereby decreasing the flexibility and strength of the globules. branches in the molecule would generate side chains that would almost certainly make it difficult to pack the cellulose molecules into microfibrils, thereby increasing the rigidity and strength of the microfibrils. branches in the molecule would generate side chains that would almost certainly make it difficult to pack the cellulose molecules into globules, thereby increasing the flexibility and strength of the globules. branches in the molecule would generate side chains that would almost certainly make it difficult to pack the cellulose molecules into microfibrils, thereby decreasing the rigidity and strength of the microfibrils.
Answers: 1

Chemistry, 22.06.2019 10:00
Water's surface tension and heat storage capacity are accounted for by its a) orbitals b) weight c) hydrogen bonds d) mass e) size
Answers: 2

Chemistry, 22.06.2019 13:00
Is 9 correct? and can someone me with 10? it’s due tomorrow, you
Answers: 1
You know the right answer?
Determine the equilibrium constant, Kp, for the following reaction, by using the two reference equat...
Questions

Health, 30.06.2019 09:00




Health, 30.06.2019 09:00



History, 30.06.2019 09:00

Mathematics, 30.06.2019 09:00

Mathematics, 30.06.2019 09:00

English, 30.06.2019 09:00




Mathematics, 30.06.2019 09:00

Biology, 30.06.2019 09:00


Chemistry, 30.06.2019 09:00


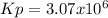
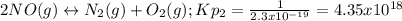
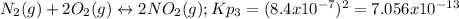
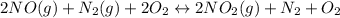
![Kp=\frac{[N_2][O_2]}{[NO]^2} * \frac{[NO_2]^2}{[N_2][O_2]^2} =Kp_2*Kp_3=4.35x10^{18}*7.056x10^{-13}=3.07x10^6](/tpl/images/0549/1890/b915d.png)


