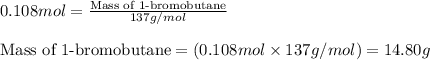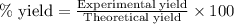Chemistry, 16.03.2020 20:53 kfloyd6046
A student isolated 7.2 g of 1-bromobutane reacting equimolar amounts of 1-butanol (10 ml) and NaBr (11.1 g) in the presence of sulfuric acid. The yield of the reaction is Select one: a. 95 % b. 48 % c. 84% d. 72 %

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:30
14. complete and balance the equations for the single displacement reactions. a. zn + pb(no3)2 -> b. al + niso4 -> 15. complete and balance the equations for the double displacement reactions. a. agno3(aq) + nacl(aq) -> b. mg(no3)2(aq) + koh(aq) -> 16. complete and balance the equations for the combustion reactions. a. __ ch4 + o2 -> b. __ c3h6 + o2 -> c. + o2 ->
Answers: 2

Chemistry, 22.06.2019 04:00
Drag each label to the correct location on the chart. classify each reaction as endothermic or exothermic.
Answers: 1

Chemistry, 22.06.2019 07:00
How far is the region from the equator or control climate
Answers: 1

Chemistry, 22.06.2019 10:00
Americium-241 undergoes fission to produce three neutrons per fission event. if a neutron-absorbing material is mixed in with this sample so that the rate of neutron production drops down to 1.8 neutrons per fission event, which will be effective at achieving a critical mass? check all that apply. remove a deflective shield surrounding the sample. remove absorbent material mixed in with the sample. compress the sample of americium-241.
Answers: 1
You know the right answer?
A student isolated 7.2 g of 1-bromobutane reacting equimolar amounts of 1-butanol (10 ml) and NaBr (...
Questions



Mathematics, 11.10.2019 03:00



Social Studies, 11.10.2019 03:00

Mathematics, 11.10.2019 03:00

World Languages, 11.10.2019 03:00

Mathematics, 11.10.2019 03:00

English, 11.10.2019 03:00

History, 11.10.2019 03:00

Computers and Technology, 11.10.2019 03:00

History, 11.10.2019 03:00

History, 11.10.2019 03:00


Mathematics, 11.10.2019 03:00

Geography, 11.10.2019 03:00



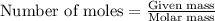 .....(1)
.....(1)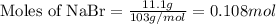
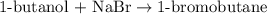
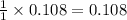 moles of 1-bromobutane
moles of 1-bromobutane