Chemistry, 16.03.2020 20:30 Tyrant4life
Many portable gas heaters and grills use propane, C3H8(g). Using enthalpies of formation, calculate the quantity of heat produced when 13.0 g of propane is completely combusted in air under standard conditions. Assume that liquid water is forming. Express the heat in kilojoules to three significant digits.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 19:10
Astudent completes a titration by adding 12.0 milliliters of naoh(aq) of unknown concentration to 16.0 milliliters of 0.15 m hcl(aq). what is the molar concentration of the naoh(aq)? 1)5.0 m 2)0.20 m 3)0.11 m 4)1.1 m
Answers: 1

Chemistry, 23.06.2019 10:30
Ethyl alcohol, also known as ethanol, has a density of 0.79 g/ml. what is the volume, in quarts, of 1.95 kg of this alcohol?
Answers: 2

Chemistry, 23.06.2019 13:30
Determine the osmotic pressure at 25 °c of an aqueous solution that is 0.028 m nano3. a) 0.685 atm b) 0.0729 atm c) 1.37 atm d) 0.0364 atm e) 2.06 atm
Answers: 2

Chemistry, 23.06.2019 16:00
Which is the best metal to use in an alloy to increase its electrical conductivity?
Answers: 2
You know the right answer?
Many portable gas heaters and grills use propane, C3H8(g). Using enthalpies of formation, calculate...
Questions

English, 14.10.2019 03:30

Mathematics, 14.10.2019 03:30

Mathematics, 14.10.2019 03:30




Biology, 14.10.2019 03:30

Geography, 14.10.2019 03:30


Biology, 14.10.2019 03:30



Computers and Technology, 14.10.2019 03:30






Mathematics, 14.10.2019 03:30

![\Delta H^o_{rxn}=\sum [n\times \Delta H_f_{(product)}]-\sum [n\times \Delta H_f_{(reactant)}]](/tpl/images/0549/0754/e893d.png)
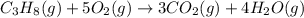
![\Delta H_{rxn}=[(3\times \Delta H_f_{(CO_2(g))})+(4\times \Delta H_f_{(H_2O(g))})]-[(1\times \Delta H_f_{(C_3H_8(g))})+(5\times \Delta H_f_{(O_2(g))})]](/tpl/images/0549/0754/b4bd0.png)
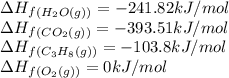
![\Delta H_{rxn}=[(3\times (-393.51))+(4\times (-241.82))]-[(1\times (-103.8))+(3\times (0))]\\\\\Delta H_{rxn}=-2044.01kJ/mol](/tpl/images/0549/0754/08c86.png)
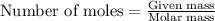
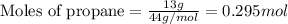
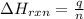
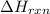 = enthalpy change of the reaction = -2044.01 kJ/mol
= enthalpy change of the reaction = -2044.01 kJ/mol