
Chemistry, 16.03.2020 20:09 ginareyes0423
Calculate ΔHrxnΔHrxn for the following reaction: 3C(s)+4H2(g)→C3H8(l)3C(s)+4H2(g)→C3 H8(l) Use the following reactions and given ΔHΔH values: C3H8(l)+5O2(g)→3CO2(g)+4H2O(g),ΔHC( s)+O2(g)→CO2(g),ΔH2H2(g)+O2(g)→2H2O (g),ΔH===−2026.6kJ−393.5kJ−483.5kJ

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 15:20
An alloy contains 66 g of pure zinc. what is the percentage of zinc in the alloy? express your answer to two significant figures and include the appropriate units.
Answers: 1

Chemistry, 22.06.2019 15:30
Which of the following are correct values for the ideal gas laws constant r
Answers: 1

Chemistry, 22.06.2019 21:30
If 22.5 of nitrogen at 748 mm hg are compressed to 725 mm hg at constant temperature. what is the new volume?
Answers: 1
You know the right answer?
Calculate ΔHrxnΔHrxn for the following reaction: 3C(s)+4H2(g)→C3H8(l)3C(s)+4H2(g)→C3 H8(l) Use the f...
Questions



English, 19.10.2021 14:00

Health, 19.10.2021 14:00


Mathematics, 19.10.2021 14:00



Mathematics, 19.10.2021 14:00

Arts, 19.10.2021 14:00






Mathematics, 19.10.2021 14:00



Mathematics, 19.10.2021 14:00

History, 19.10.2021 14:00

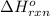 for the reaction is -120.9 kJ.
for the reaction is -120.9 kJ.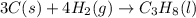
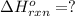
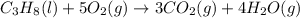
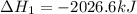
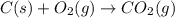
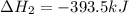 ( × 3)
( × 3)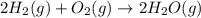
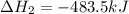 ( × 2)
( × 2)![\Delta H^o_{rxn}=[1\times (-\Delta H_1)]+[3\times \Delta H_2]+[2\times \Delta H_3]](/tpl/images/0548/9833/b4dbe.png)
![\Delta H^o_{rxn}=[(1\times -(-2026.6))+(3\times (-393.5))+(2\times (-483.5))]=-120.9kJ](/tpl/images/0548/9833/4ea2a.png)


