
Chemistry, 16.03.2020 20:05 joannachavez12345
Calculate the standard enthalpy change for the reaction at 25 ∘ C. Standard enthalpy of formation values can be found in this list of thermodynamic properties. MgCl 2 ( s ) + H 2 O ( l ) ⟶ MgO ( s ) + 2 HCl ( g )

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:40
During trial 2, what allowed you to determine that aluminum was the limiting reactant? check all that apply. all of the copper dissolved. all of the aluminum dissolved. the solution turned clear. the number of grams of copper(ii) chloride used in the reaction was greater than the number of grams of aluminum. the molar ratio of copper(ii) chloride to aluminum was greater than 3: 2, the equation’s molar ratio.
Answers: 2

Chemistry, 22.06.2019 09:20
What happened to the amount of carbon dioxide in the atmosphere from 2010–2017?
Answers: 1

Chemistry, 22.06.2019 12:30
If 22.5 liters of oxygen reacted with excess of hydrogen, how many liters of water vapor could be produced?
Answers: 3

Chemistry, 22.06.2019 14:10
Aconcentrated solution of ammonia is 14.8m and has a density of 0.899g/l. what is the concentration of ammonia in this solution in weight percent (%w/w)?
Answers: 1
You know the right answer?
Calculate the standard enthalpy change for the reaction at 25 ∘ C. Standard enthalpy of formation va...
Questions

Mathematics, 05.10.2020 16:01

Mathematics, 05.10.2020 16:01



Mathematics, 05.10.2020 16:01

Mathematics, 05.10.2020 16:01


Mathematics, 05.10.2020 16:01


History, 05.10.2020 16:01

History, 05.10.2020 16:01



Mathematics, 05.10.2020 16:01

History, 05.10.2020 16:01


Mathematics, 05.10.2020 16:01

History, 05.10.2020 16:01

Mathematics, 05.10.2020 16:01

![\Delta H^o_{rxn}=\sum [n\times \Delta H_f_{(product)}]-\sum [n\times \Delta H_f_{(reactant)}]](/tpl/images/0548/9716/e893d.png)
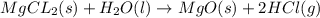
![\Delta H_{rxn}=[(1\times \Delta H_f_{(MgO(s))})+(2\times \Delta H_f_{(HCl(g))})]-[(1\times \Delta H_f_{(MgCl_2(s))})+(1\times \Delta H_f_{(H_2O(l))})]](/tpl/images/0548/9716/185a2.png)
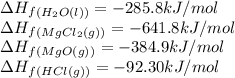
![\Delta H_{rxn}=[(1\times (-384.9))+(2\times (-92.30))]-[(1\times (-641.8))+(1\times (-285.8))]\\\\\Delta H_{rxn}=358.1kJ](/tpl/images/0548/9716/bb693.png)


