Chemistry, 16.03.2020 19:25 corbeansbrain
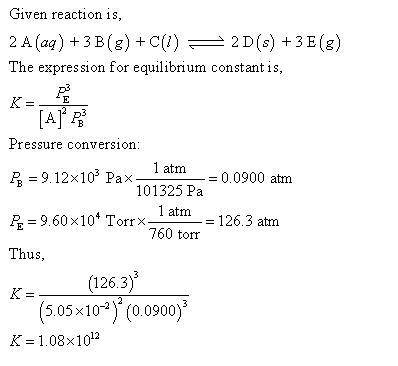
For this heterogeneous system 2 A ( aq ) + 3 B ( g ) + C ( l ) − ⇀ ↽ − 2 D ( s ) + 3 E ( g ) 2A(aq)+3B(g)+C(l)↽−−⇀2D(s)+3E(g) the concentrations and pressures at equilibrium are [ A ] = 5.66 × 10 − 2 M [A]=5.66×10−2 M , P B = 5.69 × 10 3 Pa PB=5.69×103 Pa , [ C ] = 9.14 M [C]=9.14 M , [ D ] = 15.78 M [D]=15.78 M , and P E = 9.33 × 10 4 torr PE=9.33×104 torr . Calculate the thermodynamic equilibrium constant, K K .

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 17:10
Abullet found at a crime scene may be used as evidence in a trial if the percentage of metals match to the composition of metals in a bullet from the suspect's ammunition. a forensic scientist's analysis of the bullet shows that it contains 11.9 g of lead, 0.5 g of tin, and 0.8 b of antimony. what is the percentage of lead metal in the bullet? express your answers to the one's place.
Answers: 2

Chemistry, 22.06.2019 03:30
At a temperature of 393 k, the temperature of a sample of nitrogen is 1.07 atm what will the pressure be at a temperature of 478 k
Answers: 1

Chemistry, 22.06.2019 12:00
Why are people not able to skip a dive to the deepest part of the ocean
Answers: 1

Chemistry, 23.06.2019 00:30
Titration reveals that 11.6 ml of 3.0m sulfuric acid are required to neutralize the sodium hydroxide in 25.00ml of naoh solution. what is the molarity of the naoh solution?
Answers: 1
You know the right answer?
For this heterogeneous system 2 A ( aq ) + 3 B ( g ) + C ( l ) − ⇀ ↽ − 2 D ( s ) + 3 E ( g ) 2A(aq)+...
Questions

Mathematics, 19.10.2021 14:40

Social Studies, 19.10.2021 14:40

Arts, 19.10.2021 14:40

Mathematics, 19.10.2021 14:40

History, 19.10.2021 14:40


English, 19.10.2021 14:40




Mathematics, 19.10.2021 14:50


History, 19.10.2021 14:50

Computers and Technology, 19.10.2021 14:50


Business, 19.10.2021 14:50



SAT, 19.10.2021 14:50

Mathematics, 19.10.2021 14:50