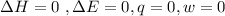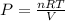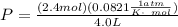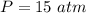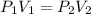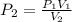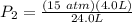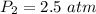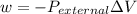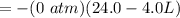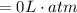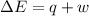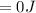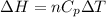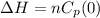Consider 2.4 moles of a gas contained in a 4.0 L bulb at a constant temperature of 32°C. This bulb is connected to an evacuated 20.0 L sealed bulb via a valve. Assume that the temperature remains constant. a.What should happen to the gas when you open the valve?b. Calculate ΔH, ΔE, q, and w for the process you described above.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 22:20
How do cfcs cause ozone depletion? how do cfcs cause ozone depletion? ultraviolet radiation breaks down cfcs, molecules containing chlorine. chlorine then breaks one oxygen atom away from ozone, leaving behind a paired oxygen molecule. ultraviolet radiation breaks down cfcs, molecules containing chlorine. chlorine then breaks two oxygen atoms away from ozone, leaving behind a paired oxygen molecule. ultraviolet radiation creates cfcs, molecules containing chlorine. chlorine then breaks two oxygen atoms away from ozone, leaving behind a paired oxygen molecule. ultraviolet radiation creates cfcs, molecules containing chlorine. chlorine then breaks one oxygen atom away from ozone, leaving behind a paired oxygen molecule.
Answers: 2

Chemistry, 23.06.2019 00:20
Which diagram represents the phase tha occurs after a solid melts?
Answers: 1

Chemistry, 23.06.2019 03:30
How many grams of sodium chloride are in 250ml of a 2.5m naci solution
Answers: 1
You know the right answer?
Consider 2.4 moles of a gas contained in a 4.0 L bulb at a constant temperature of 32°C. This bulb i...
Questions

Physics, 06.01.2020 12:31

Mathematics, 06.01.2020 12:31

Social Studies, 06.01.2020 12:31

Biology, 06.01.2020 12:31

Mathematics, 06.01.2020 12:31

Computers and Technology, 06.01.2020 12:31

Social Studies, 06.01.2020 12:31


Mathematics, 06.01.2020 12:31









Mathematics, 06.01.2020 12:31

Geography, 06.01.2020 12:31

Mathematics, 06.01.2020 12:31