
Chemistry, 16.03.2020 18:56 pierrezonra
An unknown weak acid, HA, it titrated with 1.2 M NaOH. The pH at the halfway point of this titration was found to be 4.081. If the initial pH of the weak acid solution (before titration) has a pH of 2.348, what was the concentration of the weak acid solution

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 11:00
Problem page combustion of hydrocarbons such as pentane ( c5 h12 ) produces carbon dioxide, a "greenhouse gas." greenhouse gases in the earth's atmosphere can trap the sun's heat, raising the average temperature of the earth. for this reason there has been a great deal of international discussion about whether to regulate the production of carbon dioxide.(a) write a balanced chemical equation, including physical state symbols, for the combustion of liquid pentane into gaseous carbon dioxide and gaseous water. (b) suppose 0.350 kg of pentane are burned in air at a pressure of exactly 1 atm and a temperature of 20.0 degree c. calculate the volume of carbon dioxide gas that is produced.be sure your answer has the correct number of significant digits.
Answers: 2

Chemistry, 22.06.2019 17:00
Astable electron arrangement for an atom is one that does not easily change. how is this arrangement arrived at? a. valence electrons are transferred or shared to create a full outer shell of electrons. b. valence electrons are discarded into space to create a full outer shell of electrons. c. protons (positive charge) pair with valence electrons (negative charge) to create a strong bond. d. outer shells with valence electrons are transferred or shared.
Answers: 2

Chemistry, 22.06.2019 20:30
From the choices provided below, list the reagent(s) in order that will react with cyclopentanone to form the compound shown below.
Answers: 2

Chemistry, 22.06.2019 21:00
How many neutrons does an element have if its atomic number is 50 and its mass number is 166
Answers: 1
You know the right answer?
An unknown weak acid, HA, it titrated with 1.2 M NaOH. The pH at the halfway point of this titration...
Questions

Mathematics, 10.10.2021 03:00


History, 10.10.2021 03:00



History, 10.10.2021 03:00

Mathematics, 10.10.2021 03:00

English, 10.10.2021 03:00



Biology, 10.10.2021 03:00



Mathematics, 10.10.2021 03:00


History, 10.10.2021 03:00


Mathematics, 10.10.2021 03:00


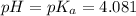 (At halfway point)
(At halfway point)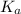 , we use the equation:
, we use the equation: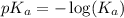
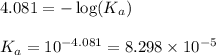
![pH=-\log [H^+]](/tpl/images/0548/8236/37e81.png)
![2.348=-\log [H^+]](/tpl/images/0548/8236/8ea67.png)
![[H^+]=10^{-2.348}=4.487\times 10^{-3}](/tpl/images/0548/8236/4ae47.png)
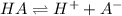
![K_a=\frac{[H^+][A^-]}{[HA]}](/tpl/images/0548/8236/66f51.png)
![[H^+]=[A^-]=4.487\times 10^{-3}](/tpl/images/0548/8236/32c77.png)
![8.298\times 10^{-5}=\frac{(4.487\times 10^{-3})\times (4.487\times 10^{-3})}{[HA]}](/tpl/images/0548/8236/82702.png)
![[HA]=0.243M](/tpl/images/0548/8236/b588d.png)


