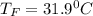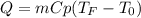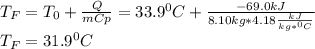Chemistry, 16.03.2020 18:59 cameron12502
A chemical reaction takes place inside a flask submerged in a water bath. The water bath contains 8.10kg of water at 33.9 degrees celsius . During the reaction 69.0kJ of heat flows out of the bath and into the flask.
Required:
Calculate the new temperature of the water bath. You can assume the specific heat capacity of water under these conditions is 4.18J*g*K. Round your answer to 3 significant digits.

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 12:20
Which is an example of the practical pursuit of alchemy? a. forming perfect substances. b. transforming base metals. c. developing metalworking techniques. d. linking spiritual characteristics with material substances.
Answers: 1

Chemistry, 22.06.2019 22:30
Essay-alternative energy sources research sources of energy that are being developed. write a report of 350-400 words discussing the information you learned concerning the development of various energy sources and the impact that you think they will have on your life. include sources cited at the end of your report using the mla format. follow the rubric guidelines. note that wikipedia is not an appropriate resource for a research paper. worth 99
Answers: 3

Chemistry, 23.06.2019 01:30
Adirect relationship can be represented by: a curve a pie chart
Answers: 2
You know the right answer?
A chemical reaction takes place inside a flask submerged in a water bath. The water bath contains 8....
Questions

History, 09.12.2020 23:50

Chemistry, 09.12.2020 23:50







Mathematics, 09.12.2020 23:50

Mathematics, 09.12.2020 23:50

Chemistry, 09.12.2020 23:50

Mathematics, 09.12.2020 23:50

Mathematics, 09.12.2020 23:50


Health, 09.12.2020 23:50

Chemistry, 09.12.2020 23:50

Chemistry, 09.12.2020 23:50


Mathematics, 09.12.2020 23:50