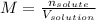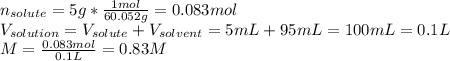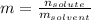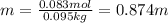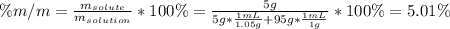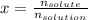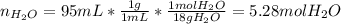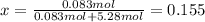Chemistry, 16.03.2020 18:22 cupcake122016
A bottle of commercial vinegar contains 5% acetic acid, ch3cooh, by volume (95% water). the density of acetic acid is 1.05 g/ml and water is 1.00 g/ml. from this data calculate the concentration of acetic acid in vinegar in: molality, molarity, parts by mass, and the mole fraction.

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 06:30
Three cards with holes are arranged in a straight line. a light is shined through the first card’s hole and travels through all three cards. what does this tell you about light rays? a) that light is reflected b) that light is refractive c) that light travels in a straight line d) that light does not travel in a straight line
Answers: 1

Chemistry, 22.06.2019 08:30
Joan writes four numbers on the board in standard form, and then she writes their scientific notation
Answers: 1

You know the right answer?
A bottle of commercial vinegar contains 5% acetic acid, ch3cooh, by volume (95% water). the density...
Questions

Mathematics, 22.08.2019 19:30

Mathematics, 22.08.2019 19:30




History, 22.08.2019 19:30





Mathematics, 22.08.2019 19:30

Mathematics, 22.08.2019 19:30