Chemistry, 16.03.2020 18:27 21cassitsh
The pH of a water is measured to be 7.5. The system is open to atmosphere and the temperature is 25 oC. Assume that the system is in equilibrium with atmosphere, calculate the concentrations of carbonic acid, bicarbonate, carbonate, and CT (total carbonates). (given pKa1 and pKa2 of H2CO3 are 6.35 and 10.33, respectively).

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:00
If the density of water is 1.0 g/cm3, which of these materials would float in water, based on their densities? check all that apply. aluminum cork iron lead wax
Answers: 1

Chemistry, 22.06.2019 21:00
Acandle’s wick is the fabric string that holds the flame, and it burns down at a constant slow pace when the candle is lit. the wick is usually surrounded by wax. which is the most important property of covalent compounds that makes them useful for making candle wax? a low boiling point a low melting point a high boiling point a high melting point
Answers: 1


You know the right answer?
The pH of a water is measured to be 7.5. The system is open to atmosphere and the temperature is 25...
Questions

Mathematics, 10.12.2020 19:40



Social Studies, 10.12.2020 19:40







Mathematics, 10.12.2020 19:40

Mathematics, 10.12.2020 19:40



Mathematics, 10.12.2020 19:40


Arts, 10.12.2020 19:40

Mathematics, 10.12.2020 19:40


Physics, 10.12.2020 19:40

![pK_{a1}=-\log[K_{a1}]](/tpl/images/0548/7437/2883e.png)
![6.35=-\log[K_{a1}]](/tpl/images/0548/7437/549c6.png)
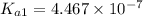
![pK_{a2}=-\log[K_{a2}]](/tpl/images/0548/7437/15d25.png)
![10.33=-\log[K_{a2}]](/tpl/images/0548/7437/898fd.png)
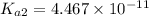
![pH=\log[H^+]](/tpl/images/0548/7437/cd7c9.png)
![7.5=\log[H^+]](/tpl/images/0548/7437/732b6.png)
![[H^+]=3.162\times 10^{-8} M](/tpl/images/0548/7437/5c72c.png)
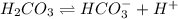
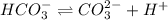
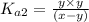
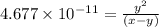 ..[1]
..[1]![x+y=[H^+]](/tpl/images/0548/7437/89af1.png)
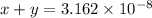 ...[2]
...[2]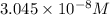
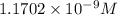
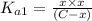
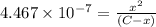
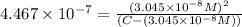
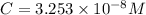
![[H_2CO_3]=(C-x)=3.253\times 10^{-8} M-3.045\times 10^{-8} M](/tpl/images/0548/7437/527d5.png)
![[H_2CO_3]=2.08\times 10^{-9} M](/tpl/images/0548/7437/67449.png)
![[CO_3^{2-}]=y=1.1702\times 10^{-9} M](/tpl/images/0548/7437/122ff.png)
![[HCO_3^{-}]=(x-y)=3.045\times 10^{-8} M-1.1702\times 10^{-9} M=2.928\times 10^{-8} M](/tpl/images/0548/7437/788be.png) Total carbonates:[TC]
Total carbonates:[TC]![[TC]=[H_2CO_3]+[HCO_3^{-}]+[CO_3^{2-}]=C](/tpl/images/0548/7437/a0d4d.png)