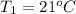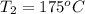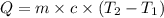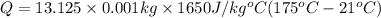Chemistry, 16.03.2020 18:16 deidaralove90
How much energy must be added to a bowl of 125popcorn kernels in order for them to reach a popping temperature of 175°C? Assume that their initial temperature is 21°C, that the specific heat capacity of popcorn is 1650 J/kg•°C, and that each kernel has a mass of 0.105 g.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 14:00
Calculate the energy required to ionize a hydrogen atom to an excited state where the electron is initially in the n = 5 energy level. report your answer in kilojoules
Answers: 1

Chemistry, 22.06.2019 15:50
Elements in group 2 are all called alkaline earth metals. what is most similar about the alkaline earth metals?
Answers: 1

Chemistry, 22.06.2019 16:50
Answer asap need by wednesday morning calculate the ph of 0.16m ch3cooh which has ka = 1.74 x 10-5 mol dm-3 best answer will be brainliest
Answers: 3

Chemistry, 23.06.2019 04:00
How many liters of water can be produced from 5.0liters of butane gas at stp, assuming excess oxygen? c4h10(g) + 02(g) → co2 (e) + h2o (g)
Answers: 2
You know the right answer?
How much energy must be added to a bowl of 125popcorn kernels in order for them to reach a popping t...
Questions


Biology, 13.11.2020 09:30

Mathematics, 13.11.2020 09:30

Mathematics, 13.11.2020 09:30

Health, 13.11.2020 09:30




Mathematics, 13.11.2020 09:30

Mathematics, 13.11.2020 09:30


Mathematics, 13.11.2020 09:30

Mathematics, 13.11.2020 09:30

Mathematics, 13.11.2020 09:30

Mathematics, 13.11.2020 09:30

World Languages, 13.11.2020 09:30


History, 13.11.2020 09:30