
Chemistry, 16.03.2020 17:31 Savannahh8503
The formation constant* of [M(CN) 4 ]2− is 7.70 × 10 16 , where M is a generic metal. A 0.150 mole quantity of M(NO3)2 is added to a liter of 0.820 M NaCN solution. What is the concentration of M2+ ions at equilibrium?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 16:50
Which of the following is an indication that a substance has undergone a chemical change? a. no new product has been formed. b. the color of the substance has not changed. c. the original constitute has not changed. d. the molecular structure has changed.
Answers: 1

Chemistry, 22.06.2019 20:00
The volume of a single vanadium atom is 9.29×10-24 cm3. what is the volume of a vanadium atom in microliters?
Answers: 3

Chemistry, 23.06.2019 04:31
Use the drop-down menus to label each of the following changes p for physical change and c for chemical change. the substance changes to a new substance. the original substance can be recovered. the color changes. gas is produced and given off. the substance changes size, shape, or volume.
Answers: 2

Chemistry, 23.06.2019 05:30
Awhite powder is added to a solution. the images show observations made before the powder is added, just after the powder has been added, and a little while later. (the liquid in the small beaker is phenol red solution.) what evidence shows that a chemical change has taken place?
Answers: 1
You know the right answer?
The formation constant* of [M(CN) 4 ]2− is 7.70 × 10 16 , where M is a generic metal. A 0.150 mole q...
Questions



Mathematics, 21.01.2021 16:20


Mathematics, 21.01.2021 16:20

Mathematics, 21.01.2021 16:20

Advanced Placement (AP), 21.01.2021 16:20


Mathematics, 21.01.2021 16:20


Mathematics, 21.01.2021 16:20

Social Studies, 21.01.2021 16:20

Mathematics, 21.01.2021 16:20


English, 21.01.2021 16:20

Social Studies, 21.01.2021 16:20

Mathematics, 21.01.2021 16:20


Mathematics, 21.01.2021 16:20

Mathematics, 21.01.2021 16:20

![M(NO_3)_2+NaCN\leftrightarrow [M(CN)_4]^{-2}+NaNO_3](/tpl/images/0548/6085/c1450.png)
![M^{+2}+4CN^-\leftrightarrow [M(CN)_4]^{-2}](/tpl/images/0548/6085/1b38d.png)
![K_F=\frac{[[M(CN)_4]^{-2}]_{eq}}{[M^{+2}]_{eq}[CN^{-}]_{eq}^4}](/tpl/images/0548/6085/aa364.png)
![[M^{+2}]_0=0.150M;[CN^-]_0=0.820M](/tpl/images/0548/6085/52301.png)
 due to the reaction progress:
due to the reaction progress: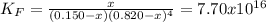
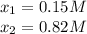
![[M^{+2}]_{eq}=0.15M-0.15M=0M](/tpl/images/0548/6085/e5b91.png)


