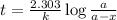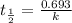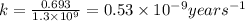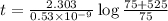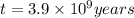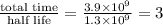Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:30
Use examples from the article to explain one positive and one negative effect that chemistry has had on society.
Answers: 2

Chemistry, 22.06.2019 06:30
How many moles of carbon dioxide will form if 2.5 moles of c3h8 is burned
Answers: 1

Chemistry, 23.06.2019 01:30
What happens to the concentration of hydronium ions as the ph of a solution increases? a. hydronium ion concentration stays the same b. hydronium ion concentration decreases c. hydronium ion concentration increases
Answers: 1

Chemistry, 23.06.2019 06:50
The student repeated the experiment using a higher concentration of acid. the same volume of acid and the same mass of magnesium ribbon were used. what volume of hydrogen gas would have been produced after 60 seconds?
Answers: 1
You know the right answer?
Potassium-40 is radioactive and decays into Argon-40. The half-life of Potassium-40 is 1.3 billion y...
Questions


English, 02.04.2020 20:07



Mathematics, 02.04.2020 20:07

English, 02.04.2020 20:07





Mathematics, 02.04.2020 20:07





Mathematics, 02.04.2020 20:07


History, 02.04.2020 20:07


Mathematics, 02.04.2020 20:07