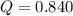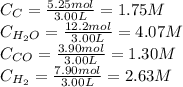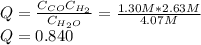The following reaction was carried out in a 3.00 L reaction vessel at 1100 K:
C(s)+H2O(...

Chemistry, 16.03.2020 16:41 lizzet2557
The following reaction was carried out in a 3.00 L reaction vessel at 1100 K:
C(s)+H2O(g) --> CO(g)+H2(g)
If during the course of the reaction, the vessel is found to contain 5.25 mol of C, 12.2 mol of H2O, 3.90 mol of CO, and 7.90 mol of H2, what is the reaction quotient Q?

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 04:30
Use the periodic table to determine the electron configuration of dysprosium (dy) and americium (am) in noble-gas notation.
Answers: 1

Chemistry, 22.06.2019 10:00
3. how much energy in joules is required to evaporate .0005 kg of liquid ammonia to vapor at the same temperature? 4. how much energy ( in megajoules ) is given up by .75 kg of water at 0c when it freezes to form ice at 0c? 5. explain how heat works between and at critical temperatures?
Answers: 2

Chemistry, 22.06.2019 18:10
Consider the following reaction at equilibrium: c(s)+h2o(g)⇌co(g)+h2(g) predict whether the reaction will shift left, shift right, or remain unchanged upon each of the following disturbances. a) c is added to the reaction mixture. b) h2ois condensed and removed from the reaction mixture c) co is added to the reaction mixture d) h2 is removed from the reaction mixture.
Answers: 3
You know the right answer?
Questions

English, 02.09.2020 22:01

English, 02.09.2020 22:01

Mathematics, 02.09.2020 22:01

Mathematics, 02.09.2020 22:01

Biology, 02.09.2020 22:01

English, 02.09.2020 22:01

English, 02.09.2020 22:01



Mathematics, 02.09.2020 22:01






Mathematics, 02.09.2020 22:01

Social Studies, 02.09.2020 22:01

Mathematics, 02.09.2020 22:01

Advanced Placement (AP), 02.09.2020 22:01