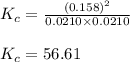Chemistry, 16.03.2020 16:29 jennemylesp19oy5
Suppose that 0.1000 mole each of H2and I2are placed in a 1.000-L flask, stoppered, and the mixture is heated to 425oC. At equilibrium, the concentration of I2is found to be 0.0210 M. Calculate Kcfor the following reaction at 425oC. H2(g) + I2(g) ⇄2 HI(g)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 17:00
Complete each row of the table below by filling in the missing prefix or missing exponent.
Answers: 1

Chemistry, 22.06.2019 21:00
What is the chemical formula for the compound formed between sodium and flour one
Answers: 1

Chemistry, 22.06.2019 22:30
Which of the following is not an assumption that scientists must make about the natural world? a. regularity b. causality c. predictability d. plausibility
Answers: 1

Chemistry, 23.06.2019 00:10
In as 1°, 2°, 3°, or 4°. be to . : °b: °c: °d: ° : °b: °c: °d: ° : °b: °c: °d: °e: °f: °g: °h: ° : °b: °c: °d: °e: °f: °g: °h: °i: °
Answers: 3
You know the right answer?
Suppose that 0.1000 mole each of H2and I2are placed in a 1.000-L flask, stoppered, and the mixture i...
Questions


Mathematics, 09.11.2020 22:40


Mathematics, 09.11.2020 22:40

History, 09.11.2020 22:40


Mathematics, 09.11.2020 22:40





History, 09.11.2020 22:40

Mathematics, 09.11.2020 22:40

Mathematics, 09.11.2020 22:40



Mathematics, 09.11.2020 22:40


Chemistry, 09.11.2020 22:40

Mathematics, 09.11.2020 22:40

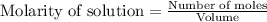
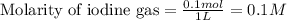
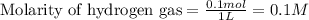
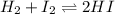
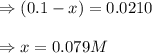
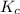 for above equation follows:
for above equation follows:![K_c=\frac{[HI]^2}{[H_2][I_2]}](/tpl/images/0548/4996/62646.png)
![[HI]_{eq}=2x=(2\times 0.079)=0.158M](/tpl/images/0548/4996/cad3c.png)
![[H_2]_{eq}=(0.1-x)=(0.1-0.079)=0.0210M](/tpl/images/0548/4996/029c8.png)
![[I_2]_{eq}=0.0210M](/tpl/images/0548/4996/0be20.png)