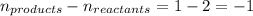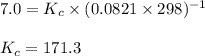The equilibrium between NO2 and N2O4 can be described by the following equation:
2NO2(g...

Chemistry, 16.03.2020 16:11 maddie53116
The equilibrium between NO2 and N2O4 can be described by the following equation:
2NO2(g) ⇌ N2O4(g) Kp = 7.0
If a sealed flask contains 1.5 atm of NO2 and 14.2 atm of N2O4. Calculate the value of Kc for the reaction,

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Read these sentences from the text. near the equator, the tropics receive the most rain on a consistent basis. as a result, the fresh water falling into the ocean decrease the salinity of the surface water in that region. [. .] . . as the salt content of sea water increases, so does its density. what can you infer about how rain affects the density of surface water near the equator?
Answers: 1

Chemistry, 22.06.2019 01:30
Reaction rate depends on how many molecules are coming into contact with each other with enough energy to react. increasing the temperature of the reactants will increase -
Answers: 3

Chemistry, 22.06.2019 13:00
Jose and eric were given four samples in lab. the results of their analysis are shown in the table. based on the data they collected, which sample is most likely a metal?
Answers: 1

Chemistry, 22.06.2019 13:10
What type of interaction occurs between the r groups of valine and isoleucine in a tertiary structure? view available hint(s) what type of interaction occurs between the r groups of valine and isoleucine in a tertiary structure? salt bridge disulfide bridge hydrogen bond hydrophobic interaction
Answers: 1
You know the right answer?
Questions

Mathematics, 29.01.2021 20:10

Mathematics, 29.01.2021 20:10

Mathematics, 29.01.2021 20:10

Spanish, 29.01.2021 20:10

History, 29.01.2021 20:10


Mathematics, 29.01.2021 20:10

Spanish, 29.01.2021 20:10

Mathematics, 29.01.2021 20:10



English, 29.01.2021 20:10

Mathematics, 29.01.2021 20:10

Engineering, 29.01.2021 20:10



Chemistry, 29.01.2021 20:10

Computers and Technology, 29.01.2021 20:10


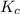 for given reaction is 171.3
for given reaction is 171.3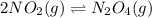
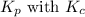 is given by the formula:
is given by the formula: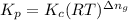
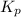 = equilibrium constant in terms of partial pressure = 7.0
= equilibrium constant in terms of partial pressure = 7.0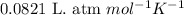
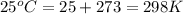
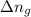 = change in number of moles of gas particles =
= change in number of moles of gas particles =