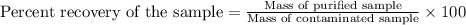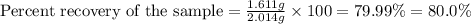Chemistry, 14.03.2020 03:22 bighomie28
You have a sample of contaminated benzoic acid that weighs 2.014 g. After performing a proper recrystallization with proper drying, the mass of your purified benzoic acid is 1.611 g. What is your percent recovery rounded to the nearest tenths

Answers: 1


Another question on Chemistry


Chemistry, 21.06.2019 18:40
Determine the energy released per kilogram of fuel used. given mev per reaction, calculate energy in joules per kilogram of reactants. consider 1 mole of tritium plus 1 mole of deuterium to be a mole of “reactions” (total molar mass = 5 grams).
Answers: 1

Chemistry, 22.06.2019 21:30
In science class richard learns that a substance has a boiling point of 230 fahrenheit his teacher ask him to convert this temperature to degrees celsius what is the boiling point of his substance in degrees celsius
Answers: 3

You know the right answer?
You have a sample of contaminated benzoic acid that weighs 2.014 g. After performing a proper recrys...
Questions

Mathematics, 14.01.2021 22:10




Social Studies, 14.01.2021 22:10

Biology, 14.01.2021 22:10


English, 14.01.2021 22:10

Mathematics, 14.01.2021 22:10

Mathematics, 14.01.2021 22:10

Mathematics, 14.01.2021 22:10

Mathematics, 14.01.2021 22:10


English, 14.01.2021 22:10

English, 14.01.2021 22:10

Arts, 14.01.2021 22:10

Mathematics, 14.01.2021 22:10

Mathematics, 14.01.2021 22:10

Mathematics, 14.01.2021 22:10