PLZ HELP, GIVING BRAINLIEST!!
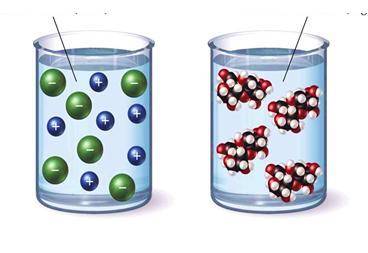
In class, students were given the pictures below and asked to pi...

Chemistry, 14.03.2020 00:29 cxttiemsp021
PLZ HELP, GIVING BRAINLIEST!!
In class, students were given the pictures below and asked to pick a solution that would conduct electricity and to justify their choice.
Based on the model above, which student's argument is correct?
A. Student B claims that the left beaker contains a covalent compound because the solute breaks apart into charged particles.
B. Student D claims to identify the solute as either ionic or covalent more information is needed than what is provided in the model.
C. Student C claims that the right beaker contains an ionic compound because the solute stays together when dissolved.
D. Student A claims that the left beaker contains an ionic compound because the solute breaks apart into charged particles.

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 14:30
Select all that apply. using a value of ksp = 1.8 x 10-2 for the reaction pbcl2 (s) pb+2(aq) + 2cl -(aq). the concentration of the products yield a ksp of 2.1 x 10-2:
Answers: 2

Chemistry, 22.06.2019 17:40
How much heat is added if 0.814g of water increase in temperature by 0.351 degree c?
Answers: 3

Chemistry, 22.06.2019 18:40
What is one real world example of a colligative property?
Answers: 2
You know the right answer?
Questions

English, 23.09.2019 23:30

Mathematics, 23.09.2019 23:30

Mathematics, 23.09.2019 23:30


World Languages, 23.09.2019 23:30

Mathematics, 23.09.2019 23:30

English, 23.09.2019 23:30

History, 23.09.2019 23:30


Chemistry, 23.09.2019 23:30


Mathematics, 23.09.2019 23:30

Social Studies, 23.09.2019 23:30

Mathematics, 23.09.2019 23:30



Mathematics, 23.09.2019 23:30


Biology, 23.09.2019 23:30

History, 23.09.2019 23:30



