Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:30
The following reaction shows sodium carbonate reacting with calcium hydroxide. na2co3 + ca(oh)2 → naoh + caco3 how many grams of naoh are produced from 20.0 grams of na2co3? (molar mass of na = 22.989 g/mol, c = 12.01 g/mol, o = 15.999 g/mol, ca = 40.078 g/mol, h = 1.008 g/mol) 12.2 grams 15.1 grams 24.4 grams 30.2 grams
Answers: 2

Chemistry, 22.06.2019 18:30
How many moles of bromine are needed to produce 3.23 moles of potassium bromide
Answers: 1

Chemistry, 22.06.2019 21:20
40dm3 of gas at 760 torr are heated from 5°c to 50°c what is the new volume
Answers: 3

Chemistry, 23.06.2019 01:30
Select the correct answer from each drop-down menu. to make a table of the elements, dmitri mendeleev sorted the elements according to their . he then split the list of elements into several columns so that elements beside each other had similar .
Answers: 2
You know the right answer?
If 1.785 g of ethanol (CHCHOH) is burned in a constant volume calorimeter causing a temperature incr...
Questions

Health, 02.03.2021 06:20



Mathematics, 02.03.2021 06:20

History, 02.03.2021 06:20

Mathematics, 02.03.2021 06:20


Mathematics, 02.03.2021 06:20


Mathematics, 02.03.2021 06:20


Mathematics, 02.03.2021 06:20

Mathematics, 02.03.2021 06:20


Mathematics, 02.03.2021 06:20

Mathematics, 02.03.2021 06:20



Mathematics, 02.03.2021 06:20

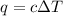
 = change in temperature = 4.32°C
= change in temperature = 4.32°C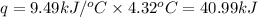
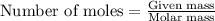
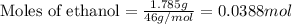
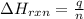
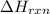 = enthalpy change of the reaction
= enthalpy change of the reaction