
Chemistry, 13.03.2020 20:03 LilFreaky666
Consider these reactions, where M represents a generic metal.
2 M (s) + 6 HCl (aq) ⟶ 2 MCl₃ (aq) + 3 H₂ (g); ΔH₁ = − 878.0 k J
HCl (g) ⟶ HCl (aq); ΔH₂ = − 74.8 k J
H₂ (g) + Cl₂ (g) ⟶ 2 HCl (g); ΔH₃ = − 1845.0 k J
MCl₃ (s) ⟶ MCl₃ ( aq ); ΔH₄ = − 497.0 k J
Use the given information to determine the enthalpy of the reaction
2 M (s) + 3 Cl₂ (g) ⟶ 2 MCl₃ (s).

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:50
Consider the reaction: n2(g) + o2(g) ? 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3

Chemistry, 22.06.2019 22:20
Asuspension of yeast cells is being grown under anaerobic conditions such that glucose is degraded to ethanol and carbon dioxide. if one wishes to follow this process by monitoring the release of 14co2, at which positions in the glucose molecule would the 14c label need to be incorporated?
Answers: 2

Chemistry, 23.06.2019 01:00
What type of chemical bond is formed between two atoms of bromine 1. metallic 2. hydrogen 3. ionic 4. covalent
Answers: 1

Chemistry, 23.06.2019 03:30
If you need to add 27.50ml of a solution, which piece of glassware would you use to deliver this volume and explain how you would determine if the 27.50 ml was measured?
Answers: 2
You know the right answer?
Consider these reactions, where M represents a generic metal.
2 M (s) + 6 HCl (aq) ⟶ 2 MCl₃ (...
2 M (s) + 6 HCl (aq) ⟶ 2 MCl₃ (...
Questions

English, 01.07.2019 05:20



History, 01.07.2019 05:20

History, 01.07.2019 05:20


Mathematics, 01.07.2019 05:20

Geography, 01.07.2019 05:20

Mathematics, 01.07.2019 05:20

Mathematics, 01.07.2019 05:20

Chemistry, 01.07.2019 05:20

Mathematics, 01.07.2019 05:20

Physics, 01.07.2019 05:20

Mathematics, 01.07.2019 05:20

Mathematics, 01.07.2019 05:20


Mathematics, 01.07.2019 05:20

Mathematics, 01.07.2019 05:20


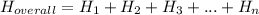 .
.


