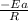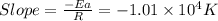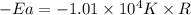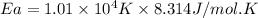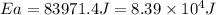Answers: 2


Another question on Chemistry


Chemistry, 23.06.2019 01:00
The primary products of complete combustion of fossil fuels are a. carbon dioxide and water b. methane and water c. carbon monoxide and water d. carbon dioxide and carbon monoxide
Answers: 1

Chemistry, 23.06.2019 01:30
Ascientist conducted an experiment and discovered that certain plants grow faster when given a particular amount of fertilizer. anouther scientist conducted the same experiment and got similar results. which concept does this best illustrate? a) repetition b) replication c) precision d) validity
Answers: 2

Chemistry, 23.06.2019 03:40
The following questions a24 - a26 relate to 100 ml of 0.0150 m solution of benzoic acid (c6h3cooh). ka(c6h3cooh) = 6.4 x 10^-5. what is the ph of the solution after the addition of 1 x 10^-3 moles of naoh? you may assume no volume change to the solution upon addition of the naoh.
Answers: 2
You know the right answer?
The rate consnt for a reaction is measured as a function of temperature. A plot of ln k versus 1/T i...
Questions


Mathematics, 08.04.2021 01:30



History, 08.04.2021 01:30

Mathematics, 08.04.2021 01:30

Biology, 08.04.2021 01:30



Mathematics, 08.04.2021 01:30


Physics, 08.04.2021 01:30

Social Studies, 08.04.2021 01:30

Mathematics, 08.04.2021 01:30




Mathematics, 08.04.2021 01:30


Mathematics, 08.04.2021 01:30

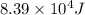
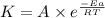
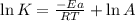
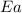 = activation energy for the reaction
= activation energy for the reaction