
Chemistry, 13.03.2020 17:28 ramentome7542
You have an aqueous solution of chromium(III) nitrate that you titrate with an aqueous solution of sodium hydroxide. After a certain amount of titrant has been added, you observe a precipitate forming. You add more sodium hydroxide solution and the precipitate dissolves, leaving a solution again. What has happened?1) The precipitate was chromium hydroxide, which then reacted with more hydroxide to produce a soluble complex ion, Cr(OH)4?.2) The precipitate was chromium hydroxide, which dissolved once more solution was added, forming Cr3+(aq).3) The precipitate was sodium nitrate, which reacted with more nitrate to produce the soluble complex ion Na(NO3)2?.4) The precipitate was sodium hydroxide, which re-dissolved in the larger volume.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:30
The table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 3

Chemistry, 22.06.2019 14:30
Is a pencil falling to the floor anon contact force, a force, or a contact force
Answers: 1

Chemistry, 22.06.2019 17:30
Energy defines the different "states" of matter. in no more than 3 sentences, describe the amount of kinetic energy that each of the 3 states of matter possesses and relate that to the atom/molecular motion of each "state".
Answers: 2

Chemistry, 22.06.2019 19:10
Δu of , in kj/kg, as it isto k, (a)as a of , (b) at , (c) at .
Answers: 2
You know the right answer?
You have an aqueous solution of chromium(III) nitrate that you titrate with an aqueous solution of s...
Questions




Geography, 30.11.2020 06:30

Mathematics, 30.11.2020 06:30

Social Studies, 30.11.2020 06:30


Mathematics, 30.11.2020 06:30



Mathematics, 30.11.2020 06:30

Mathematics, 30.11.2020 06:30

Biology, 30.11.2020 06:30


Chemistry, 30.11.2020 06:30

Social Studies, 30.11.2020 06:30

Chemistry, 30.11.2020 06:30



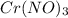 +3 NaOH →
+3 NaOH → 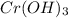 (s)+
(s)+ 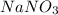
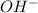 (aq) →
(aq) → 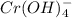 (aq)
(aq)

