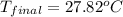Chemistry, 13.03.2020 05:01 afolmar2006
A 26.4 g sample of aluminum at 100.6 °C is added to 100.4 g of water at 22.0 °C in a constant pressure calorimeter. What is the final temperature of the water in °C? The specific heat capacity of aluminum is 0.903 J/g°C .

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:40
For each of the following compounds, write the formula then predict whether it would be a strong, weak, or non-electrolyte when placed in di water. for the ionic compounds only, put (s) or (aq) after the forrmula formula strong, weak or non electrolyte? a calcium hydroxide b. silver carbonate c. lead(ii) sulfate d. phosphorus trifluoride e. sodium phosphide f barium sulfate g. strontium acetate h. zinc nitrate
Answers: 3


Chemistry, 22.06.2019 14:30
How can carbon move from "land" to bodies of water? describe the way human impact has lead to increased levels of co2 in the atmosphere.
Answers: 2

Chemistry, 22.06.2019 21:30
The solid xy decomposes into gaseous x and y: xy(s) m x(g) + y(g) kp = 4.1 (at 0 °c) if the reaction is carried out in a 22.4 l container, which initial amounts of x and y will result in the formation of solid xy?
Answers: 1
You know the right answer?
A 26.4 g sample of aluminum at 100.6 °C is added to 100.4 g of water at 22.0 °C in a constant pressu...
Questions



Mathematics, 11.10.2019 14:30

History, 11.10.2019 14:30

Mathematics, 11.10.2019 14:30


Mathematics, 11.10.2019 14:30

Social Studies, 11.10.2019 14:30


Business, 11.10.2019 14:30










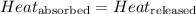
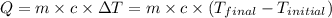
![m_1\times c_1\times (T_{final}-T_1)=-[m_2\times c_2\times (T_{final}-T_2)]](/tpl/images/0546/2501/09236.png) ......(1)
......(1)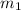 = mass of aluminium = 26.4 g
= mass of aluminium = 26.4 g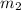 = mass of water = 100.4 g
= mass of water = 100.4 g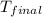 = final temperature = ?°C
= final temperature = ?°C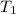 = initial temperature of aluminium = 100.6°C
= initial temperature of aluminium = 100.6°C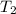 = initial temperature of water = 22.0°C
= initial temperature of water = 22.0°C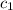 = specific heat of aluminium = 0.903 J/g°C
= specific heat of aluminium = 0.903 J/g°C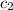 = specific heat of water= 4.186 J/g°C
= specific heat of water= 4.186 J/g°C![26.4\times 0.903\times (T_{final}-100.6)=-[100.6\times 4.186\times (T_{final}-23.7)]](/tpl/images/0546/2501/b8661.png)