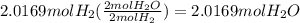27. For the reaction 2 H2 + O2 → 2 H20, how
many moles of H2O would you get from 2.0169
...


Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:30
What must happen before a body cell can begin mitotic cell division
Answers: 1

Chemistry, 22.06.2019 06:00
Which of the following did jj thompson discover about atoms? a)an atom has an internal structure. b) atoms are tiny indivisible particles. c)electrons orbit the nucleus of an atom. d) the nucleus of an atom contains protons and neutrons.
Answers: 2

Chemistry, 22.06.2019 16:00
What rule is used to determine how many covalent bonds an element can form? a. the number of covalent bonds is equal to six c the number of covalent bonds is equal to five minus the group number plus the group number b. the number of covalent bonds is equal to eight d. none of the above minus the group number select the best answer from the choices provided
Answers: 2

Chemistry, 22.06.2019 16:10
Amixture initially contains a, b, and c in the following concentrations: [a] = 0.300 m , [b] = 1.05 m , and [c] = 0.550 m . the following reaction occurs and equilibrium is established: a+2b⇌c at equilibrium, [a] = 0.140 m and [c] = 0.710 m . calculate the value of the equilibrium constant, kc.
Answers: 1
You know the right answer?
Questions






Physics, 18.01.2022 21:20

SAT, 18.01.2022 21:20

Mathematics, 18.01.2022 21:30

Physics, 18.01.2022 21:30




SAT, 18.01.2022 21:30





Mathematics, 18.01.2022 21:30

English, 18.01.2022 21:30

Mathematics, 18.01.2022 21:30

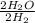 . The twos reduce, making this a one-to-one ratio.
. The twos reduce, making this a one-to-one ratio.