
Chemistry, 13.03.2020 04:31 Penelope9687
An iron bar weighed 565 g. After the bar had been standing in moist air for a month, exactly one-eighth of the iron turned to rust (Fe2O3). Calculate the final mass of the iron bar and rust.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 13:00
Read the given expression. x = number of protons − number of core electrons which of the following explains the identity of x and its trends across a period? x is the effective nuclear charge, and it remains constant across a period. x is the screening constant, and it remains constant across a period. x is the effective nuclear charge, and it increases across a period. x is the screening constant, and it increases across a period.
Answers: 1

Chemistry, 21.06.2019 20:30
If 10.g of agno3 is available, what volume of 0.25 m agno3 can be prepared
Answers: 1

Chemistry, 22.06.2019 12:00
Which of the following units is not an official si unit? mole liter kilogram ampere
Answers: 1

You know the right answer?
An iron bar weighed 565 g. After the bar had been standing in moist air for a month, exactly one-eig...
Questions


Mathematics, 23.11.2020 01:20

Mathematics, 23.11.2020 01:20

Mathematics, 23.11.2020 01:20

Mathematics, 23.11.2020 01:20

Mathematics, 23.11.2020 01:20

Mathematics, 23.11.2020 01:20

Mathematics, 23.11.2020 01:20

Mathematics, 23.11.2020 01:20



Social Studies, 23.11.2020 01:20


History, 23.11.2020 01:20




Mathematics, 23.11.2020 01:20


Spanish, 23.11.2020 01:20

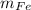 ) = 565 ×
) = 565 × 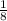 = 70.625 g
= 70.625 g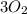 →
→ 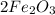
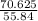 = 1.26 mol
= 1.26 mol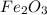 = 159.68 g
= 159.68 g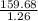 = 126.730 g
= 126.730 g 

