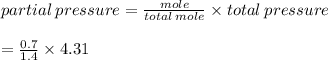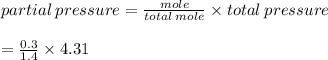Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 12:50
what is the density of an object that has a mass of 10 g and a volumeof 5 ml? a. 0.5 g/ mlb. 2 g/mlc. 15 g/ mld. 50 g/ ml
Answers: 1

Chemistry, 21.06.2019 13:00
How are ionic bonds formed and what is the attractive force within an ionic bond
Answers: 1

Chemistry, 21.06.2019 22:00
To save time, you can approximate the initial mass of the solid to the nearest ±1 g. for example, if you are asked to add 14.3 g of copper, add between 13 g and 15 g. which of the following sets include two samples with an equal density? which all that apply below 15.4 g gold and 18.7 g silver 15.2 g copper and 50.0 g copper 20.2 g silver and 20.2 g copper 11.2 g gold and 14.9 g gold
Answers: 1

Chemistry, 22.06.2019 02:00
If you add 10ml of hot water to 10ml of cold water and the change in tempature 8°c calculate how much energy is gained by the cold water
Answers: 1
You know the right answer?
A gas mixture contains 0.700 mol of N2, 0.300 mol of H2, and 0.400 mol of CH4. Calculate the pressur...
Questions



Mathematics, 25.03.2020 00:12


Mathematics, 25.03.2020 00:12





Biology, 25.03.2020 00:12

Mathematics, 25.03.2020 00:12


Mathematics, 25.03.2020 00:12





Chemistry, 25.03.2020 00:12