Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:00
What type of electromagnetic radiation has a shorter wavelength than blue light
Answers: 2

Chemistry, 22.06.2019 21:20
One way in which the useful metal copper is produced is by dissolving the mineral azurite, which contains copper(ii) carbonate, in concentrated sulfuric acid. the sulfuric acid reacts with the copper(ii) carbonate to produce a blue solution of copper(ii) sulfate. scrap iron is then added to this solution, and pure copper metal precipitates out because of the following chemical reaction: (s) (aq) (s) (aq) suppose an industrial quality-control chemist analyzes a sample from a copper processing plant in the following way. he adds powdered iron to a copper(ii) sulfate sample from the plant until no more copper will precipitate. he then washes, dries, and weighs the precipitate, and finds that it has a mass of .
Answers: 2

Chemistry, 23.06.2019 04:00
The movement of tectonic plates and in two locations is described below: location a: tectonic played push together location b: tectonic plates push apart
Answers: 1

Chemistry, 23.06.2019 05:30
The term gas is limited to those substances that exist in the gaseous state at
Answers: 1
You know the right answer?
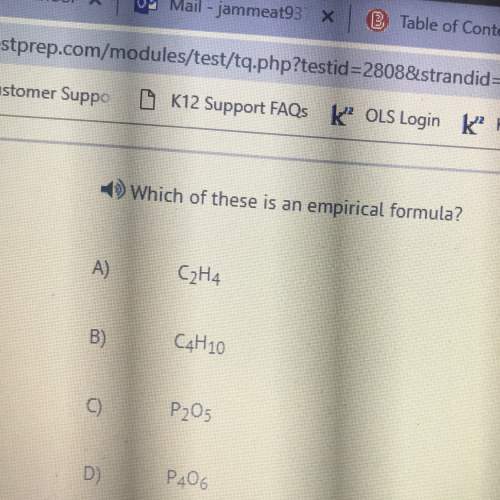
From the change in the P2O5 trap mass before and after reaction, determine the mass of H2O produced...
Questions


Physics, 04.12.2020 05:30

Mathematics, 04.12.2020 05:30

Mathematics, 04.12.2020 05:30

Mathematics, 04.12.2020 05:30

History, 04.12.2020 05:30

Computers and Technology, 04.12.2020 05:30


English, 04.12.2020 05:30

English, 04.12.2020 05:30


Arts, 04.12.2020 05:30


Mathematics, 04.12.2020 05:30

Social Studies, 04.12.2020 05:30

Chemistry, 04.12.2020 05:30

Mathematics, 04.12.2020 05:30

English, 04.12.2020 05:30