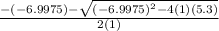Chemistry, 12.03.2020 21:40 daniellaZemira
Suppose a 250.0 mL flask is filled with 1.3 mol of I2 and 1.0 mol of HI. The following reaction becomes possible:
H2 (g) +I2 (g) ⇆ 2HI (g)
The equilibrium constant for this reaction is 0.983 at the temperature of the flask.
Calculate the equilibrium molarity of HI. Round your answer to two decimal places.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 08:30
The mass of a neutron is equal to the mass of a proton plus the mass of an electron. true or false false true
Answers: 1

Chemistry, 22.06.2019 15:50
How many moles of potassium hydroxide are needed to completely react with 2.94 moles of aluminum sulfate
Answers: 1

Chemistry, 22.06.2019 17:00
The atoms of a solid aluminum can are close together, vibrating in a rigid structure. if the can is warmed up on a hot plate, what happens to the atoms?
Answers: 3

Chemistry, 22.06.2019 22:00
Scientists often have to deal with numbers that are either very large or very small. for example, the radius of the sun is approximately 696,000 kilometers, while bacterial cells are as small as 1.9 × 10-4 millimeters. express each number in an alternate form.
Answers: 1
You know the right answer?
Suppose a 250.0 mL flask is filled with 1.3 mol of I2 and 1.0 mol of HI. The following reaction beco...
Questions


Mathematics, 05.01.2020 12:31


Mathematics, 05.01.2020 12:31




Mathematics, 05.01.2020 12:31

Geography, 05.01.2020 12:31


Social Studies, 05.01.2020 12:31


Social Studies, 05.01.2020 12:31


Mathematics, 05.01.2020 12:31



Mathematics, 05.01.2020 12:31


Biology, 05.01.2020 12:31

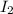 = 1.3 mol
= 1.3 mol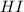 = 1.0 mole
= 1.0 mole
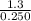
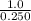
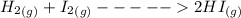
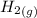 +
+ 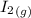 ----->
-----> 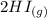
![K = \frac{[HI]^2}{[H_2][I_2]}](/tpl/images/0545/4972/78f4e.png)
![K = \frac{[4-2x]^2}{[x][5.20+x]}](/tpl/images/0545/4972/b30d0.png) where K = 0.983
where K = 0.983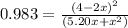
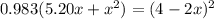
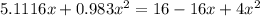
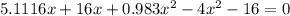
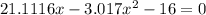
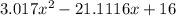
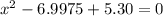
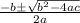
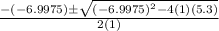
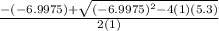 OR
OR