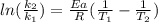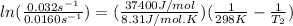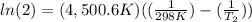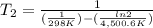Chemistry, 12.03.2020 20:03 shelbylynn17
The activation energy of a certain reaction is 37.4 kJ/mol . At 25 ∘C , the rate constant is 0.0160s−1 . At what temperature in degrees Celsius would this reaction go twice as fast?

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 03:00
Which of these would be caused by a chemical change? a) the formation of lava. b) sedimantary rock layering over time. c) metamorphic rock forming from igneous. d) metamorphic rock eroding to form sedimentary rock.
Answers: 3

Chemistry, 22.06.2019 12:30
A50.0 ml sample of gas at 20.0 atm of pressure is compressed to 40.0 atm of pressure at constant temperature. what is the new volume? 0.0100 ml 0.325 ml 25.0 ml 100. ml
Answers: 1

Chemistry, 22.06.2019 22:30
Calculate the concentration of all species in a 0.165 m solution of h2co3.
Answers: 1
You know the right answer?
The activation energy of a certain reaction is 37.4 kJ/mol . At 25 ∘C , the rate constant is 0.0160s...
Questions


Mathematics, 22.01.2021 01:00

English, 22.01.2021 01:00

Mathematics, 22.01.2021 01:00


Mathematics, 22.01.2021 01:00

Geography, 22.01.2021 01:00




Chemistry, 22.01.2021 01:00



Mathematics, 22.01.2021 01:00



Biology, 22.01.2021 01:00

Chemistry, 22.01.2021 01:00

Geography, 22.01.2021 01:00