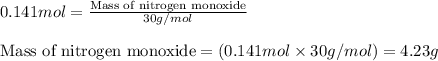Chemistry, 12.03.2020 17:28 kailinaguilar2187
For the following reaction, 5.62 grams of oxygen gas are mixed with excess ammonia. The reaction yields 3.58 grams of nitrogen monoxide. ammonia (g) oxygen (g) nitrogen monoxide (g) water (g) What is the ideal yield of nitrogen monoxide

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:00
According to each substances heat of fusion, which of the items below requires more heat to be added per gram of substance to go from solid to liquid? silver sulfur water lead
Answers: 2


Chemistry, 23.06.2019 06:30
Acompound has the molecular formula c3h8. which class of organic compounds does it belong to?
Answers: 2

Chemistry, 23.06.2019 11:00
Nh4no3 n2o + 2h2o a chemist who is performing this reaction starts with 160.1 g of nh4no3. the molar mass of nh4no3 is 80.03 g/mol; the molar mass of water (h2o) is 18.01 g/mol. what mass, in grams, of h2o is produced?
Answers: 1
You know the right answer?
For the following reaction, 5.62 grams of oxygen gas are mixed with excess ammonia. The reaction yie...
Questions

History, 13.02.2021 01:00

Mathematics, 13.02.2021 01:00


Biology, 13.02.2021 01:00

Health, 13.02.2021 01:00





Chemistry, 13.02.2021 01:00

Mathematics, 13.02.2021 01:00

Mathematics, 13.02.2021 01:00

Mathematics, 13.02.2021 01:00

Mathematics, 13.02.2021 01:00

Health, 13.02.2021 01:00

Biology, 13.02.2021 01:00


Mathematics, 13.02.2021 01:00


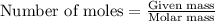 .....(1)
.....(1)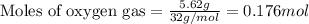
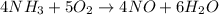
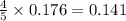 moles of nitrogen monoxide
moles of nitrogen monoxide