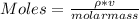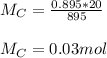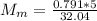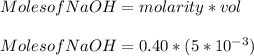You added 5.00 mL of 0.40 M NaOH in methanol to 20.00 mL of cooking oil. Calculate the number of moles of vegetable oil, methanol, and NaOH that are initially present in the sample. Assume the density of vegetable oil is 0.895 g/mL and the molar mass is 895 g/mol. Look up the density and molar mass of any other compounds as needed.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:00
Which of the following is not a true statement about dwarf planets? a the kuiper belt contains comets, asteroids, and dwarf planets. b ceres is a dwarf planet located in the kuiper belt. c the largest known dwarf planet in the solar system is named eris.
Answers: 2

Chemistry, 22.06.2019 05:50
According to coulomb's law, how would the electrical force between particles change if the product of their electrical charge increased?
Answers: 1

Chemistry, 22.06.2019 17:40
Areaction in which products can react to re-form reactants is
Answers: 1

Chemistry, 23.06.2019 07:30
Chris is about to do an experiment to measure the density of water at several temperatures. his teacher has him prepare and sign a safety contract before beginning the experiment. which term is mostlikely part of the safety contract
Answers: 3
You know the right answer?
You added 5.00 mL of 0.40 M NaOH in methanol to 20.00 mL of cooking oil. Calculate the number of mol...
Questions

Mathematics, 13.12.2021 03:30

Mathematics, 13.12.2021 03:30

Mathematics, 13.12.2021 03:30


Mathematics, 13.12.2021 03:30

Biology, 13.12.2021 03:30



Mathematics, 13.12.2021 03:30

Mathematics, 13.12.2021 03:30


History, 13.12.2021 03:30


Mathematics, 13.12.2021 03:30

Mathematics, 13.12.2021 03:30

Mathematics, 13.12.2021 03:30

Mathematics, 13.12.2021 03:30



Social Studies, 13.12.2021 03:30