
Chemistry, 12.03.2020 06:06 amohammad6
A basic solution contains the iodide and phosphate ions that are to be separated via selective precipitation. the i– concentration, which is 9.00×10-5 m, is 10,000 times less than that of the po43– ion at 0.900 m . a solution containing the silver(i) ion is slowly added. answer the questions below. ksp of agi is 8.30×10-17 and of ag3po4, 8.90×10-17.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 12:30
What is the percent composition of ca(oh)2? 37.7% ca, 53.0% o, and 10.3% h 45.5% ca, 38.2% o, and 16.3% h 54.0% ca, 43.0% o, and 2.7% h 64.7% ca, 27.0% o, and 8.3% h
Answers: 2


Chemistry, 22.06.2019 19:20
The equation picture below shows which type of nuclear reaction u 235 + n x e 134 + sr 100 + 2n
Answers: 1

Chemistry, 22.06.2019 22:00
Choose all the answers that apply. fluorine (f) has an atomic number of 9 and an atomic weight of 18.99. fluorine has a. 9 protons b. 10 neutrons c. 18 electrons d. an atomic mass of 19 e. at least one isotope
Answers: 1
You know the right answer?
A basic solution contains the iodide and phosphate ions that are to be separated via selective preci...
Questions



Chemistry, 29.08.2019 01:30

History, 29.08.2019 01:30

Business, 29.08.2019 01:30





History, 29.08.2019 01:30



English, 29.08.2019 01:30

Geography, 29.08.2019 01:30

History, 29.08.2019 01:30





Chemistry, 29.08.2019 01:30

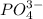 ion at 0.900 m . a solution containing the silver(i) ion is slowly added. answer the questions below. ksp of agi is 8.30×10-17 and of
ion at 0.900 m . a solution containing the silver(i) ion is slowly added. answer the questions below. ksp of agi is 8.30×10-17 and of 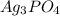 ,
, 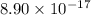 .
.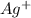 concentration required to cause precipitation of AgI.
concentration required to cause precipitation of AgI.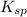 is equal to the ionic product.
is equal to the ionic product.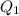 is as follows.
is as follows.![K_{sp} = [Ag^{+}][I^{-}]](/tpl/images/0544/6507/7f793.png)
![[I^{-}] = 7.7 \times 10^{-5}](/tpl/images/0544/6507/26cb8.png)
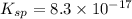
![[Ag^{+}] = \frac{k_{sp}}{I^{-}}](/tpl/images/0544/6507/fac7f.png)
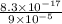
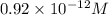
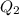 the expression for
the expression for ![K_{sp} = [Ag^{+}]^{3}[PO^{3-}_{4}]](/tpl/images/0544/6507/9aea6.png)
![[Ag^{+}] = (\frac{K_{sp}}{[PO^{3-}_{4}]})^{\frac{1}{3}}](/tpl/images/0544/6507/33b2e.png)
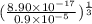
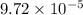 M
M 

