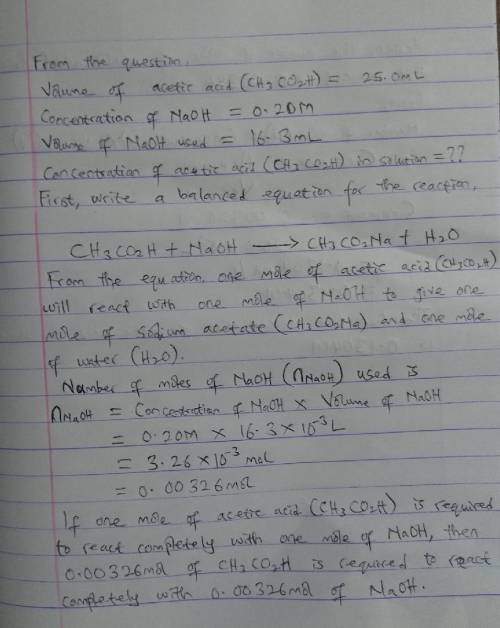Chemistry, 12.03.2020 06:08 JavyHart9695
A 25.0mL solution acetic acid (CH3CO2H) is titrated with 0.20M NaOH and reaches the endpoint after the addition of 16.3mL of NaOH. What is the concentration of acetic acid in solution

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:30
Label a-f based on the table using c for concentrated and d for dilute
Answers: 2

Chemistry, 22.06.2019 17:30
In a heat of an engine, if 700 j enters the system, and the piston does 400 j of work what is the final internal (thermal) energy of the system if the initial energy is 1,500 j
Answers: 2

Chemistry, 22.06.2019 19:30
Helium decays to form lithium. which equation correctly describes this decay?
Answers: 2

You know the right answer?
A 25.0mL solution acetic acid (CH3CO2H) is titrated with 0.20M NaOH and reaches the endpoint after t...
Questions

Mathematics, 07.06.2021 19:00



History, 07.06.2021 19:00

English, 07.06.2021 19:00


Mathematics, 07.06.2021 19:00

English, 07.06.2021 19:00

Biology, 07.06.2021 19:00




Mathematics, 07.06.2021 19:00


Mathematics, 07.06.2021 19:00



Chemistry, 07.06.2021 19:00

English, 07.06.2021 19:00