
Chemistry, 12.03.2020 05:41 Rachelmontes1
A 25-kg iron block initially at 350 oC is quenched in an insulated tank that contains 100 kg of water at 18 oC. Assuming the water that vaporizes during the process condenses back in the tank, determine the total entropy change during this process.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 17:00
Noble gases are the most reactive elements on the periodic table. a. true b. false
Answers: 2

Chemistry, 22.06.2019 06:10
How many moles of gas are present if p=11 atm, v=12l, t=185k?
Answers: 1

Chemistry, 22.06.2019 13:00
Imagine that you push on a large rock. at what point does your effort change the rock’s mechanical energy?
Answers: 1

Chemistry, 22.06.2019 14:00
The content of manganese (mn) in steel was determined spectrophotometrically and with the use of the standard addition method. an unknown sample of mn from a digested steel sample gave an absorbance of 0.185 when analyzed spectrophotometrically. when 5.00 ml of solution containing 95.5 ppm mn was added to 50.0 ml of the unknown steel solution (digested sample), the absorbance was 0.248. calculate the concentration, in parts-per-million (ppm), of mn in the digested steel sample solution.
Answers: 3
You know the right answer?
A 25-kg iron block initially at 350 oC is quenched in an insulated tank that contains 100 kg of wate...
Questions

History, 18.10.2021 05:50

Arts, 18.10.2021 05:50


English, 18.10.2021 05:50





Mathematics, 18.10.2021 06:00

Physics, 18.10.2021 06:00

Mathematics, 18.10.2021 06:00

Mathematics, 18.10.2021 06:00

Chemistry, 18.10.2021 06:00

Biology, 18.10.2021 06:00

Mathematics, 18.10.2021 06:00

Chemistry, 18.10.2021 06:00

Mathematics, 18.10.2021 06:00


Mathematics, 18.10.2021 06:00

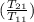 = 25 kg×0.444 J/g·°C㏑
= 25 kg×0.444 J/g·°C㏑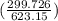 = -8124.296 J/K = -8.124 kJ/K
= -8124.296 J/K = -8.124 kJ/K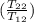 = 100 kg×4.186 J/g·°C㏑
= 100 kg×4.186 J/g·°C㏑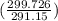 = 12152.01 J/K = 12.15201 kJ/K
= 12152.01 J/K = 12.15201 kJ/K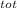 = ΔS₁ + ΔS₂ = -8.124 kJ/K + 12.15201 kJ/K = 4.03 kJ/K.
= ΔS₁ + ΔS₂ = -8.124 kJ/K + 12.15201 kJ/K = 4.03 kJ/K.

