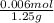Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:30
Water (2510 g ) is heated until it just begins to boil. if the water absorbs 5.09×105 j of heat in the process, what was the initial temperature of the water?
Answers: 3

Chemistry, 22.06.2019 12:30
A50.0 ml sample of gas at 20.0 atm of pressure is compressed to 40.0 atm of pressure at constant temperature. what is the new volume? 0.0100 ml 0.325 ml 25.0 ml 100. ml
Answers: 1

Chemistry, 22.06.2019 20:10
Insoluble sulfide compounds are generally black in color. which of the following combinations could yield a black precipitate? check all that apply. na2s(aq)+kcl(aq) li2s(aq)+pb(no3)2(aq) pb(clo3)2(aq)+nano3(aq) agno3(aq)+kcl(aq) k2s(aq)+sn(no3)4(aq)
Answers: 1

You know the right answer?
It takes 12.45 milliliters of 0.50 M NAOH to back titrate a mixture containing 1.25 grams of baking...
Questions





Mathematics, 26.02.2021 17:40



Health, 26.02.2021 17:40

Mathematics, 26.02.2021 17:40


Mathematics, 26.02.2021 17:40



Mathematics, 26.02.2021 17:40

Social Studies, 26.02.2021 17:40

Mathematics, 26.02.2021 17:40



Geography, 26.02.2021 17:40

 (as 1 L = 1000 ml)
(as 1 L = 1000 ml)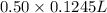 (12.45 ml = 0.1245 L)
(12.45 ml = 0.1245 L)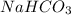 will be as follows.
will be as follows.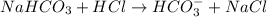
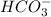 . Hence, moles of
. Hence, moles of 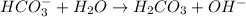
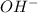 .
.