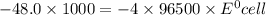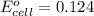Chemistry, 12.03.2020 02:06 stranger123
Calculate the standard potential, E ∘ , E°, for this reaction from its Δ G ∘ ΔG° value. X ( s ) + Y 4 + ( aq ) ⟶ X 4 + ( aq ) + Y ( s ) Δ G ∘ = − 48.0 kJ

Answers: 3


Another question on Chemistry


Chemistry, 21.06.2019 18:10
Using complete sentences, explain how to predict the products and balance the reaction between sulfuric acid and potassium hydroxide.
Answers: 1

Chemistry, 22.06.2019 03:40
In an effort to address concerns about global warming, a power plant in portland,oregon is designed to take all of its exhaust gases from its boilers and recycle the co2 using the solvay process to make sodium hydrogen carbonate. the reaction is shown below. nh3(g) + h2o(l) + co2(g) + nacl(aq) → nahco3(aq) + nh4cl(aq) how many liters each of nh3 and co2 (both at stp) would be consumed to produce 3.00 kg of sodium bicarbonate? the volume of both nh3 and co2 would be
Answers: 1

Chemistry, 22.06.2019 06:00
Why is permeable soil best for plants that need a lot of drainage?
Answers: 1
You know the right answer?
Calculate the standard potential, E ∘ , E°, for this reaction from its Δ G ∘ ΔG° value. X ( s ) + Y...
Questions


English, 05.12.2021 21:00

Mathematics, 05.12.2021 21:00




Mathematics, 05.12.2021 21:00

Mathematics, 05.12.2021 21:00

Mathematics, 05.12.2021 21:00

English, 05.12.2021 21:00

Chemistry, 05.12.2021 21:00

Mathematics, 05.12.2021 21:00

English, 05.12.2021 21:00

Mathematics, 05.12.2021 21:00

Biology, 05.12.2021 21:00


English, 05.12.2021 21:00


History, 05.12.2021 21:00

Computers and Technology, 05.12.2021 21:00

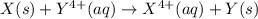
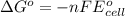
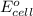 = standard cell potential = ?
= standard cell potential = ?