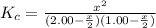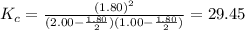Chemistry, 12.03.2020 02:05 martinbricein10
2.00 mol of H2(g) and 1.00 mol of I2(g) are placed in a 1.00 L container, and they react to form HI(g). At equilibrium, it is found that 1.80 moles of HI(g) are present in the container. Calculate K for the reaction: H2(g) + I2(g) ⇄ 2 HI(g)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:30
Electrons are extremely important to what area of technology? a) anti-aging research b) household product development c) electronics d) drug discovery
Answers: 3

Chemistry, 22.06.2019 16:10
Given the following equation: 2a1 + 3mgcl2 --> 2alcl3 + 3mg how many moles of aluminum chloride are produced from 2.5 moles of magnesium chloride?
Answers: 1

Chemistry, 23.06.2019 00:30
Nuclear decay is the spontaneous decay of one element into a. an x-ray b. a ray of light c. another element
Answers: 1

Chemistry, 23.06.2019 01:30
What is the importance of interlocking the fingers and rubbing while washing hands? the palms are the dirtiest parts of the hands. the spaces between the fingers get washed. the backs of the hands get washed. the fingernails are the dirtiest parts of the hands
Answers: 1
You know the right answer?
2.00 mol of H2(g) and 1.00 mol of I2(g) are placed in a 1.00 L container, and they react to form HI(...
Questions




Mathematics, 18.03.2021 21:00

English, 18.03.2021 21:00




Physics, 18.03.2021 21:00

Mathematics, 18.03.2021 21:00

Social Studies, 18.03.2021 21:00

Mathematics, 18.03.2021 21:00

Social Studies, 18.03.2021 21:00


Social Studies, 18.03.2021 21:00

Biology, 18.03.2021 21:00




English, 18.03.2021 21:00

![[H_2]= \frac{2.00 mol}{1.00 L}=2.00 M](/tpl/images/0544/0690/a78aa.png)
![[I_2]= \frac{I.00 mol}{1.00 L}=1.00 M](/tpl/images/0544/0690/94c40.png)
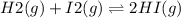
![[HI]=\frac{1.80 mol}{1.00L} = 1.80M= x](/tpl/images/0544/0690/bcad7.png)
![K_c=\frac{[HI]^2}{[H_2][I_2]}](/tpl/images/0544/0690/62646.png)