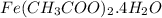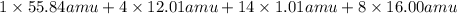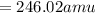Chemistry, 12.03.2020 01:36 Joshuafranklindude
Calculate the molecular (formula) mass of the compound: iron(II) acetate tetrahydrate. A. 186.9 amu B. 245.9 amu C. 191.5 amu D. 242.7 amu E. 197.9 amu

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:30
Which statement is true about this reaction? 14n+1h 15o it is a practical source of energy on earth. it occurs only outside the solar system. its product is heavier than each of its reactants. it shows the critical mass of an element.
Answers: 2

Chemistry, 22.06.2019 23:00
What is the formula of the ionic compound composed of calcium cations and chloride anions
Answers: 1

Chemistry, 23.06.2019 10:30
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 3.75 mol fe and 8.70 mol nio(oh) react?
Answers: 1

Chemistry, 23.06.2019 12:00
372 ml is the volume of aluminum, density is 2.70 g/ml what is the mass in grams
Answers: 1
You know the right answer?
Calculate the molecular (formula) mass of the compound: iron(II) acetate tetrahydrate. A. 186.9 amu...
Questions





History, 12.04.2021 20:10

Mathematics, 12.04.2021 20:10




Mathematics, 12.04.2021 20:10

Mathematics, 12.04.2021 20:10



Mathematics, 12.04.2021 20:10




Social Studies, 12.04.2021 20:10

Computers and Technology, 12.04.2021 20:10