Chemistry, 12.03.2020 00:41 ayoismeisalex
A solution of hydrochloric acid of unknown concentration was titrated with 0.10 M NaOH. If a 100.-mL sample of the HCl solution required exactly 1.0 mL of the NaOH solution to reach the equivalence point, what was the initial pH of the HCl solution

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:00
Chen drew a diagram to compare the ways in which different organisms obtain nitrogen. which label belongs to the area marked z?
Answers: 3

Chemistry, 22.06.2019 10:30
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1

Chemistry, 22.06.2019 21:30
The solid xy decomposes into gaseous x and y: xy(s) m x(g) + y(g) kp = 4.1 (at 0 °c) if the reaction is carried out in a 22.4 l container, which initial amounts of x and y will result in the formation of solid xy?
Answers: 1

You know the right answer?
A solution of hydrochloric acid of unknown concentration was titrated with 0.10 M NaOH. If a 100.-mL...
Questions


Chemistry, 27.01.2021 20:50

Mathematics, 27.01.2021 20:50







Mathematics, 27.01.2021 20:50




Spanish, 27.01.2021 20:50




English, 27.01.2021 20:50

Mathematics, 27.01.2021 20:50

Mathematics, 27.01.2021 20:50

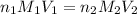
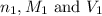 are the n-factor, molarity and volume of acid which is HCl
are the n-factor, molarity and volume of acid which is HCl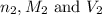 are the n-factor, molarity and volume of base which is NaOH.
are the n-factor, molarity and volume of base which is NaOH.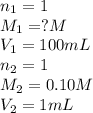
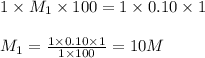
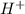 ions and 1 mole of
ions and 1 mole of 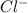 ions
ions![pH=-\log[H^+]](/tpl/images/0543/9407/cf945.png)
![[H^+]=0.001M](/tpl/images/0543/9407/58906.png)