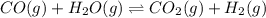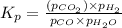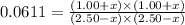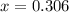Chemistry, 12.03.2020 00:02 Mitchmorgan3816
CO(g ) + H2O(g ) <---> CO2(g ) + H2(g ), Kc = 0.0611 at 2000 K . A reaction mixture initially contains a CO partial pressure of 2.50 atm, an H2O partial pressure of 2.50 atm, a CO2 partial pressure of 1.00 atm, and an H2 partial pressure of 1.00 atm at 2000 K. Calculate the equilibrium partial pressure of CO

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:30
How many moles are in 250 grams of tungsten (w)? * 4.4x10^23 moles 4.2x10^23 moles 0.7 moles 1.4 moles
Answers: 3

Chemistry, 22.06.2019 05:50
What are the 4 phases of matter in order of increasing engery content?
Answers: 2

Chemistry, 22.06.2019 11:00
Iron (3) oxide will decompose in the presence of hydrogen gas and heater to produced iron and digydrogen monoxide white a balanced chemical equation
Answers: 1

Chemistry, 22.06.2019 18:00
The human activities in two locations are described below: location a: rampant use of plastic containers location b: excessive use of pesticides and fertilizers which statement is most likely true? location a will have poor air quality because plastic is biodegradable. location a will experience water scarcity because plastic absorbs moisture. the population of honeybees will increase in location b because production of crops will increase. the population of fish in location b will decrease because the water is contaminated.
Answers: 1
You know the right answer?
CO(g ) + H2O(g ) <---> CO2(g ) + H2(g ), Kc = 0.0611 at 2000 K . A reaction mixture initially...
Questions




Mathematics, 14.04.2020 19:50

Mathematics, 14.04.2020 19:50



Social Studies, 14.04.2020 19:50



Mathematics, 14.04.2020 19:50




History, 14.04.2020 19:50



Geography, 14.04.2020 19:51

History, 14.04.2020 19:51

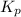 is the constant of a certain reaction at equilibrium.
is the constant of a certain reaction at equilibrium.