
Chemistry, 11.03.2020 22:54 Jaylen52709
Determine the ph of a 0.500 m hno2 solution. Ka of hno2 is 4.6 × 10−4.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 09:30
The chart shows the bid provided by four contractors to complete a job. which contractor is the most cost-effective?
Answers: 3

Chemistry, 22.06.2019 22:30
Gusing the milligrams of ascorbic acid you entered above, the ratio of total sample volume to aliquot volume, and the total milligrams of the vitamin c tablet that you dissolved, calculate the mass of ascorbic acid in the vitamin c tablet for each trial. do this by scaling up to find the amount (mg) of ascorbic acid in your 250 ml flask. enter your calculated mass of ascorbic acid in the vitamin c tablet, for each trial. be sure to enter your calculated mass in the corresponding order that you entered your milligrams of ascorbic acid. the milligrams of ascorbic acid you entered for entry #1 previously should correspond to the mass of ascorbic acid that you enter for entry #1 here.
Answers: 1

Chemistry, 23.06.2019 00:30
Ok, so i have 2 questions. try to answer them both: (the topic is fire) 1) how can you represent the chemical reaction of fire? 2) what kind of bond is formed in this chemical reaction
Answers: 3

You know the right answer?
Determine the ph of a 0.500 m hno2 solution. Ka of hno2 is 4.6 × 10−4....
Questions











History, 28.08.2020 22:01


Law, 28.08.2020 22:01


Mathematics, 28.08.2020 22:01

English, 28.08.2020 22:01




Mathematics, 28.08.2020 22:01

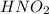 solution is 1.82
solution is 1.82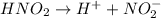
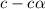
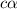
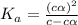
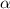 = ?
= ?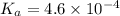
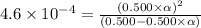
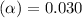
![[H^+]=c\times \alpha](/tpl/images/0543/5772/4fc41.png)
![[H^+]=0.500\times 0.030=0.015](/tpl/images/0543/5772/28636.png)
![pH=-log[H^+]](/tpl/images/0543/5772/15713.png)
![pH=-log[0.015]=1.82](/tpl/images/0543/5772/45771.png)


