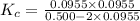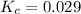Chemistry, 11.03.2020 22:49 webbjalia04
At high temperature, 2.00 mol of HBr was placed in a 4.00 L container where it decomposed in the reaction: 2HBr(g) H2(g) Br2(g) At equilibrium the concentration of Br2 was measured to be 0.0955 M. What is Kc for this reaction at this temperature

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:00
Which of the following is not a true statement about dwarf planets? a the kuiper belt contains comets, asteroids, and dwarf planets. b ceres is a dwarf planet located in the kuiper belt. c the largest known dwarf planet in the solar system is named eris.
Answers: 2

Chemistry, 22.06.2019 10:30
Astudent reacts 13 moles of iron with 21 moles of oxygen according to the following equation:
Answers: 1


Chemistry, 23.06.2019 03:40
The following questions a24 - a26 relate to 100 ml of 0.0150 m solution of benzoic acid (c6h3cooh). ka(c6h3cooh) = 6.4 x 10^-5. what is the ph of the solution after the addition of 1 x 10^-3 moles of naoh? you may assume no volume change to the solution upon addition of the naoh.
Answers: 2
You know the right answer?
At high temperature, 2.00 mol of HBr was placed in a 4.00 L container where it decomposed in the rea...
Questions

Business, 20.07.2019 13:30

Physics, 20.07.2019 13:30

Business, 20.07.2019 13:30

Mathematics, 20.07.2019 13:30



Mathematics, 20.07.2019 13:30


History, 20.07.2019 13:30

Mathematics, 20.07.2019 13:30


Biology, 20.07.2019 13:30






History, 20.07.2019 13:30


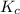 for this reaction at this temperature is 0.029
for this reaction at this temperature is 0.029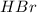 = 2.00 mole
= 2.00 mole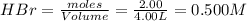
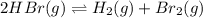
![K_c=\frac{[H_2\times [Br_2]}{[HBr]^2}](/tpl/images/0543/5528/3a3d4.png)
![[Br_2]](/tpl/images/0543/5528/f23ed.png) = x = 0.0955 M
= x = 0.0955 M