Chemistry, 11.03.2020 22:01 catboy7196
Aluminum–lithium (Al–Li) alloys have been developed by the aircraft industry to reduce the weight and improve the performance of its aircraft. A commercial aircraft skin material having a density of 2.14 g/cm3 is desired. Compute the concentration of Li (in wt%) that is required. The densities of aluminum and lithium are 2.71 and 0.534 g/cm3, respectively.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 10:30
Geothermal energy for industrial use is available almost anywhere. a.true b.false
Answers: 2

Chemistry, 22.06.2019 19:00
A4.86 g piece of metal was placed in a graduated cylinder containing 15.5 ml of water. the water level rose to 17.3 ml. what is the density of the metal. i need the steps of how to solve it to so i can use a formula to work out other problems.
Answers: 1

You know the right answer?
Aluminum–lithium (Al–Li) alloys have been developed by the aircraft industry to reduce the weight an...
Questions


Mathematics, 16.11.2020 07:20

Mathematics, 16.11.2020 07:20

Mathematics, 16.11.2020 07:20

English, 16.11.2020 07:20


History, 16.11.2020 07:20

History, 16.11.2020 07:20

Chemistry, 16.11.2020 07:20

Biology, 16.11.2020 07:20

Mathematics, 16.11.2020 07:20


English, 16.11.2020 07:20


Biology, 16.11.2020 07:20


History, 16.11.2020 07:20

Business, 16.11.2020 07:20

Mathematics, 16.11.2020 07:20

Mathematics, 16.11.2020 07:20

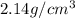
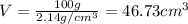
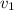
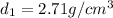
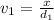
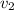
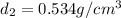
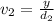
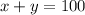 ..[1]
..[1]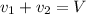
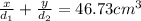
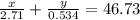 ..[2]
..[2]