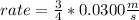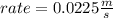A+B YIELDS C+D rate=k[A][B]^2

Chemistry, 11.03.2020 22:18 kaylag8242p2qj2e
The reaction has an initial rate of 0.0300 M/s.
A+B YIELDS C+D rate=k[A][B]^2
What will the initial rate be if [A] is halved and [B] is tripled? (answer) M/s
What will the initial rate be if [A] is tripled and [B] is halved? answer M/s

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:30
How air particles exert a pressure on the inside of the balloon
Answers: 1

Chemistry, 22.06.2019 00:30
If 3.00 g of titanium metal is reacted with 6.00 g of chlorine gas, cl2, to form 7.7 g of titanium (iv) chloride in a combination reaction, what is the percent yield of the product?
Answers: 1

Chemistry, 22.06.2019 01:30
Arollercoaster car at the top of a hill has potential energy kinetic energy chemical energy light energy
Answers: 1

Chemistry, 22.06.2019 10:30
What determines the average kinetic energy of the particles in a gas? a. the number of collisions b. the number of particles c. the size of the particles d. the temperature
Answers: 1
You know the right answer?
The reaction has an initial rate of 0.0300 M/s.
A+B YIELDS C+D rate=k[A][B]^2
A+B YIELDS C+D rate=k[A][B]^2
Questions

Biology, 26.04.2021 21:00


Business, 26.04.2021 21:00

Mathematics, 26.04.2021 21:00

Mathematics, 26.04.2021 21:00

Mathematics, 26.04.2021 21:00


Mathematics, 26.04.2021 21:00



Mathematics, 26.04.2021 21:00


Social Studies, 26.04.2021 21:00

History, 26.04.2021 21:00





Mathematics, 26.04.2021 21:00

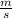
![rate=k*\frac{[A]}{2} *(3*[B])^{2}](/tpl/images/0543/4772/9b904.png)
![rate =k*\frac{[A]}{2} *9*[B]^{2}](/tpl/images/0543/4772/7e94b.png)
![rate=\frac{9}{2}* k*[A] *[B]^{2}](/tpl/images/0543/4772/813f9.png)
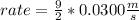
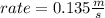
![rate=k*[A]*3 *(\frac{[B]}{2} )^{2}](/tpl/images/0543/4772/d9b35.png)
![rate=k*[A]*3*\frac{[B]^{2}}{4}](/tpl/images/0543/4772/15847.png)
![rate =\frac{3}{4}* k*[A] *[B]^{2}](/tpl/images/0543/4772/47b74.png)