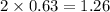At a certain temperature, the equilibrium constant, K c , for this reaction is 53.3. H 2 ( g ) + I 2 ( g ) − ⇀ ↽ − 2 HI ( g ) K c = 53.3 At this temperature, 0.800 mol H 2 and 0.800 mol I 2 were placed in a 1.00 L container to react. What concentration of HI is present at equilibrium?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:00
Which type of orbits are found in the principal energy level n = 2 a - s b - s, f c - s, d d - s, p e - s, p, d
Answers: 1

Chemistry, 22.06.2019 08:40
Which statement can best be concluded from the ideal gas law?
Answers: 2


Chemistry, 22.06.2019 19:30
Helium decays to form lithium. which equation correctly describes this decay?
Answers: 2
You know the right answer?
At a certain temperature, the equilibrium constant, K c , for this reaction is 53.3. H 2 ( g ) + I 2...
Questions

Social Studies, 22.03.2021 19:10

Mathematics, 22.03.2021 19:10

Biology, 22.03.2021 19:10




Mathematics, 22.03.2021 19:10

Mathematics, 22.03.2021 19:10



Biology, 22.03.2021 19:10


Mathematics, 22.03.2021 19:10





Mathematics, 22.03.2021 19:10

Mathematics, 22.03.2021 19:10

History, 22.03.2021 19:10

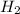 = 0.800 mole
= 0.800 mole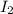 = 0.800 mole
= 0.800 mole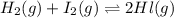
![K_c=\frac{[HI]^2}{[H_2]\times [l_2]}](/tpl/images/0543/3494/7dfaa.png)
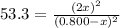
 at equilibrium = 2 x =
at equilibrium = 2 x =