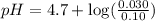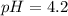Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 14:00
Calculate the frequency of a wave in a spring toy. the wave has a speed of 1.1 meters per second and a wavelength of 0.1 meters. *
Answers: 2

Chemistry, 22.06.2019 21:30
If you burn 46.6 g of hydrogen and produce 416 g of water, how much oxygen reacted
Answers: 3

Chemistry, 22.06.2019 23:30
Imagine a small synthetic vesicle made from pure phospholipids enclosing an interior lumen containing 1 mm glucose and 1 mm sodium chloride. if the vesicle is placed in pure water, which of the following happens faster? a. na+ diffuses out. b. cl– diffuses out. c. h2o diffuses in. d. glucose diffuses out. e. sodium chloride diffuses out.
Answers: 3
You know the right answer?
What is the pH of a solution prepared by mixing 30.00 mL of 0.10 MCH3CO2H with 30.00 mL of 0.030 MCH...
Questions


Mathematics, 04.02.2021 03:10

History, 04.02.2021 03:10


History, 04.02.2021 03:10


Mathematics, 04.02.2021 03:10

History, 04.02.2021 03:10




Mathematics, 04.02.2021 03:10

Health, 04.02.2021 03:10



Arts, 04.02.2021 03:10

Biology, 04.02.2021 03:10

Advanced Placement (AP), 04.02.2021 03:10


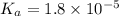
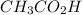 = 0.10 M
= 0.10 M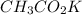 = 0.030 M
= 0.030 M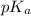 .
.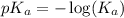
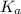 in this expression, we get:
in this expression, we get: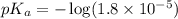
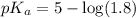
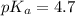
![pH=pK_a+\log \frac{[Salt]}{[Acid]}](/tpl/images/0543/3032/e961a.png)
![pH=pK_a+\log \frac{[CH_3CO_2K]}{[CH_3CO_2H]}](/tpl/images/0543/3032/8b433.png)